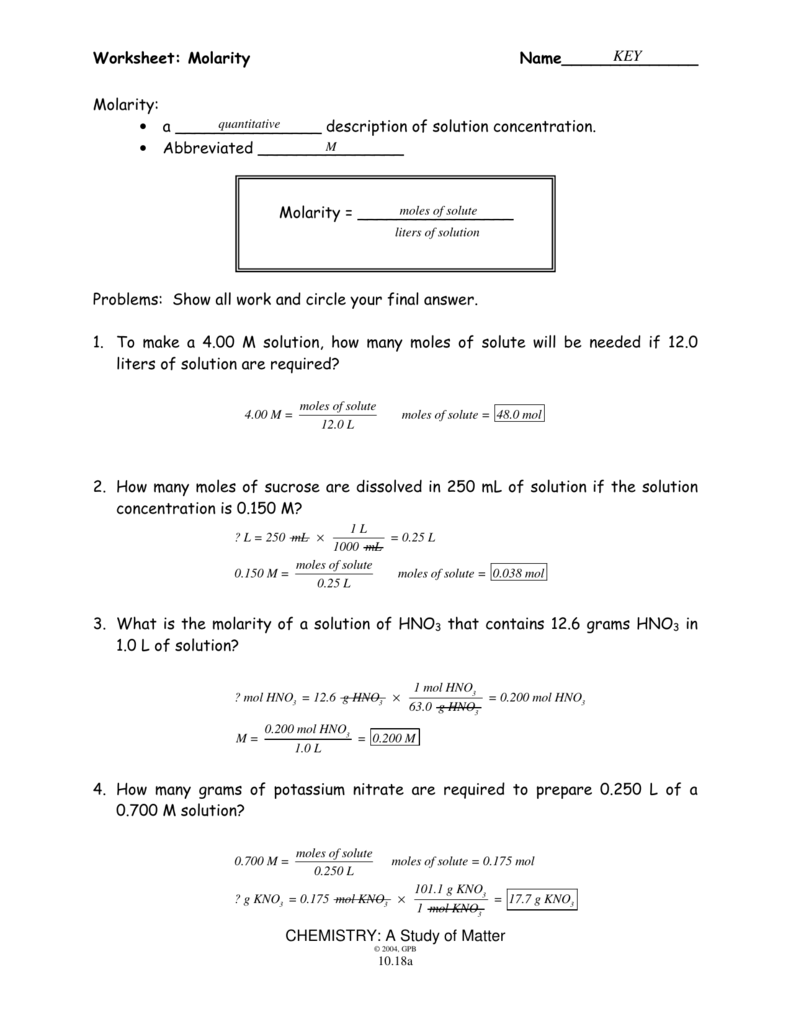
Worksheet Molarity Answers - What is the molarity of 500. Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? (x) (1.00 l) = 28.0 g /. Ml of solution made from 0.70. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? What is the molarity of the following solutions given that: What is the molarity of sodium chloride in sea water?
What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? What is the molarity of the following solutions given that: (x) (1.00 l) = 28.0 g /. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Ml of solution made from 0.70. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of 500. What is the molarity of sodium chloride in sea water?
What is the molarity of sodium chloride in sea water? What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Ml of solution made from 0.70. What is the molarity of the following solutions given that: What is the molarity of 500. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. (x) (1.00 l) = 28.0 g /.
Molarity Worksheet With Answers
Ml of solution made from 0.70. Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? What is the molarity of sodium chloride in sea water? 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.
SOLUTION Molarity worksheet for practise Studypool
What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? What is the molarity of 500. Ml of solution made from 0.70. (x) (1.00 l) = 28.0 g /. What is the molarity of sodium chloride in sea water?
Dilutions And Molarity Worksheet Molarity And Dilutions N
Ml of solution made from 0.70. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? What is the molarity of the following solutions given that: (x) (1.00 l) = 28.0 g /. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.
Molarity Worksheet With Answers
(x) (1.00 l) = 28.0 g /. Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Ml of solution made from 0.70. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.
Calculating Molarity, Moles, and Volume A Chemistry Worksheet Made
What is the molarity of the following solutions given that: Sea water contains roughly 28.0 g of nacl per liter. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. (x) (1.00 l) = 28.0 g /. What is the molarity of sodium chloride in sea water?
Molarity
What is the molarity of the following solutions given that: What is the molarity of 500. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? (x) (1.00 l) = 28.0 g /. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.
Molarity Practice Worksheet Answer Elegant Molarity Practice Worksheet
What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? What is the molarity of 500. What is the molarity of the following solutions given that: (x) (1.00 l) = 28.0 g /. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.
10++ Molarity Calculations Worksheet Worksheets Decoomo
What is the molarity of sodium chloride in sea water? Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? What is the molarity of 500. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl?
Molarity Worksheet 2 ANSWERS Google Docs
What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Sea water contains roughly 28.0 g of nacl per liter. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of sodium chloride in sea water? (x) (1.00 l) = 28.0 g /.
Chem 1020 Molarity worksheet, version 2 1. What is the molarity of a
Ml of solution made from 0.70. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of 500.
(X) (1.00 L) = 28.0 G /.
Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of sodium chloride in sea water? 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of the following solutions given that:
What Is The Molarity Of A 0.30 Liter Solution Containing 0.50 Moles Of Nacl?
What is the molarity of 500. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Ml of solution made from 0.70.