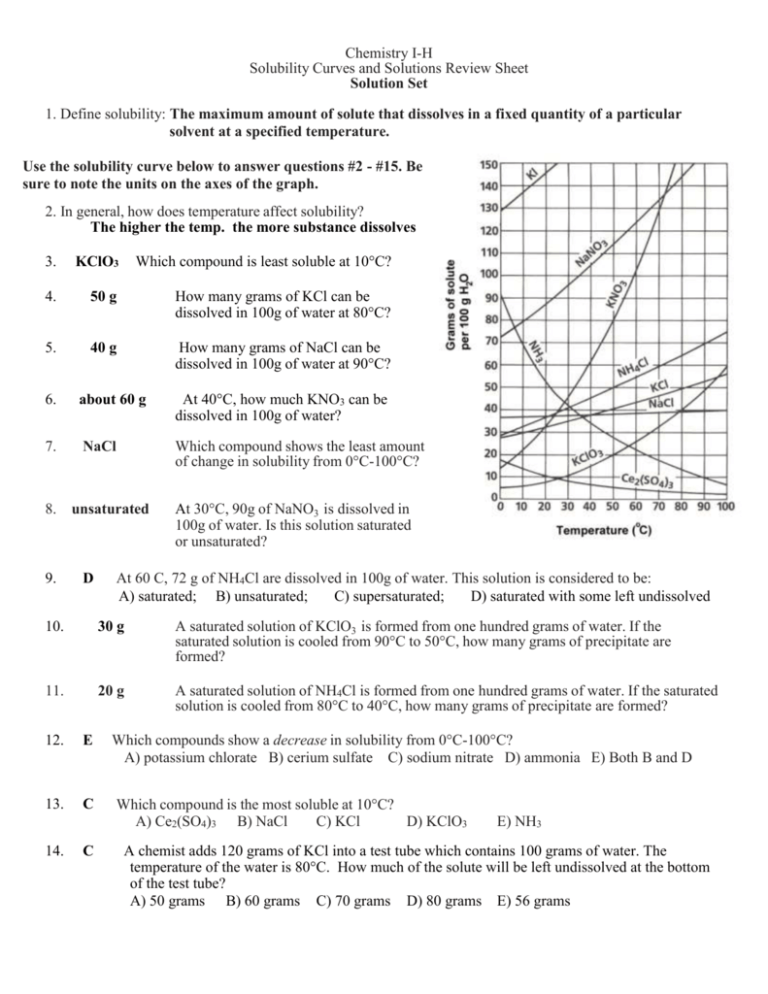
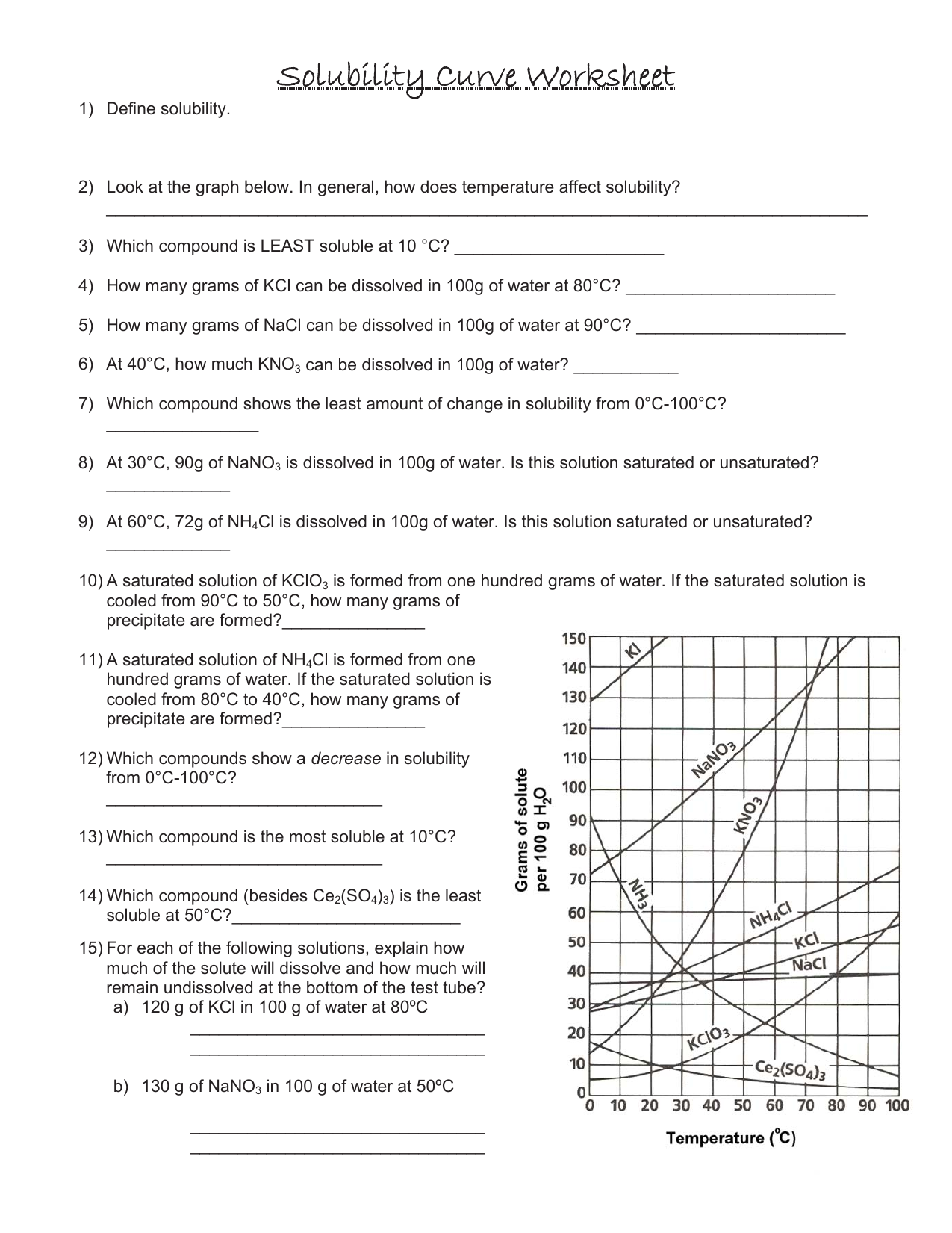
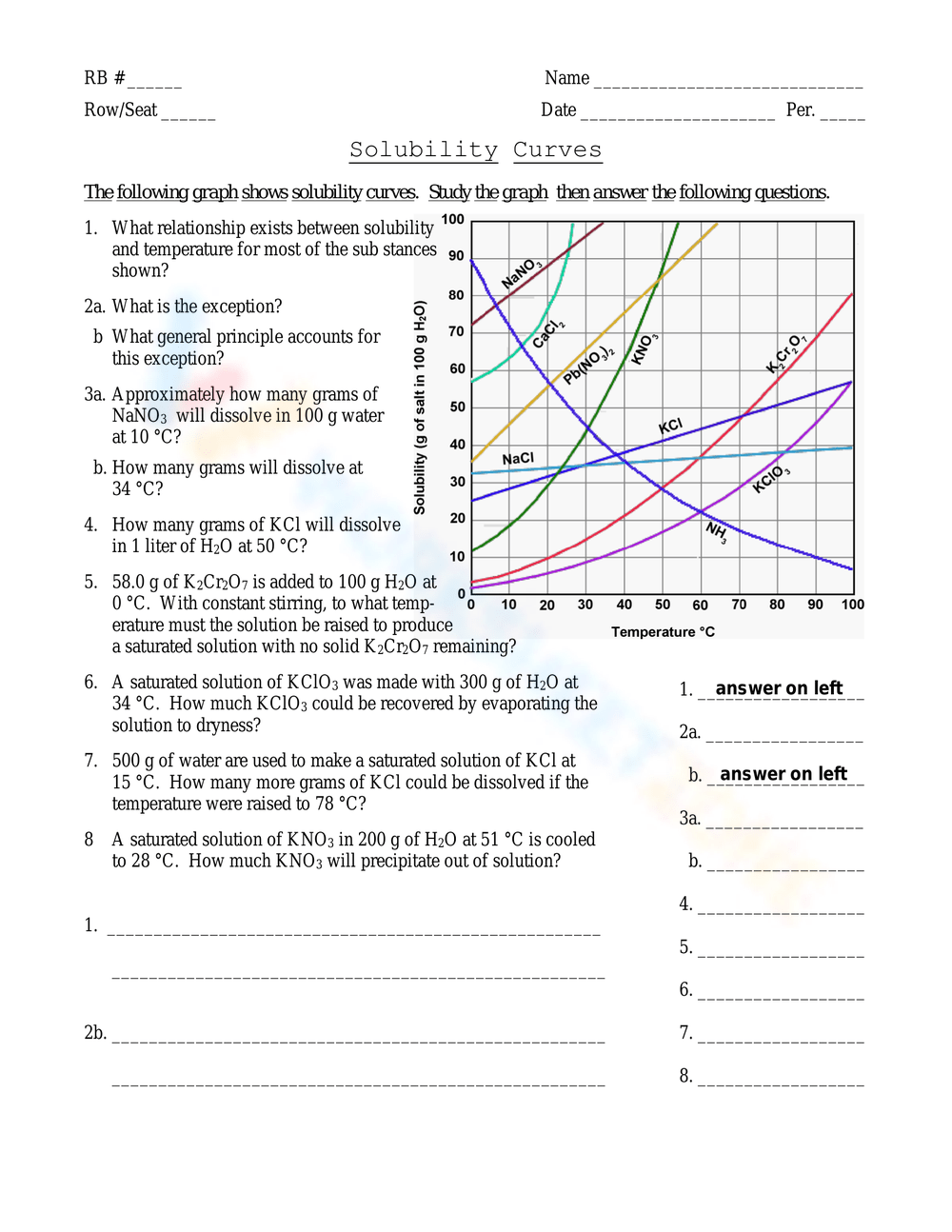
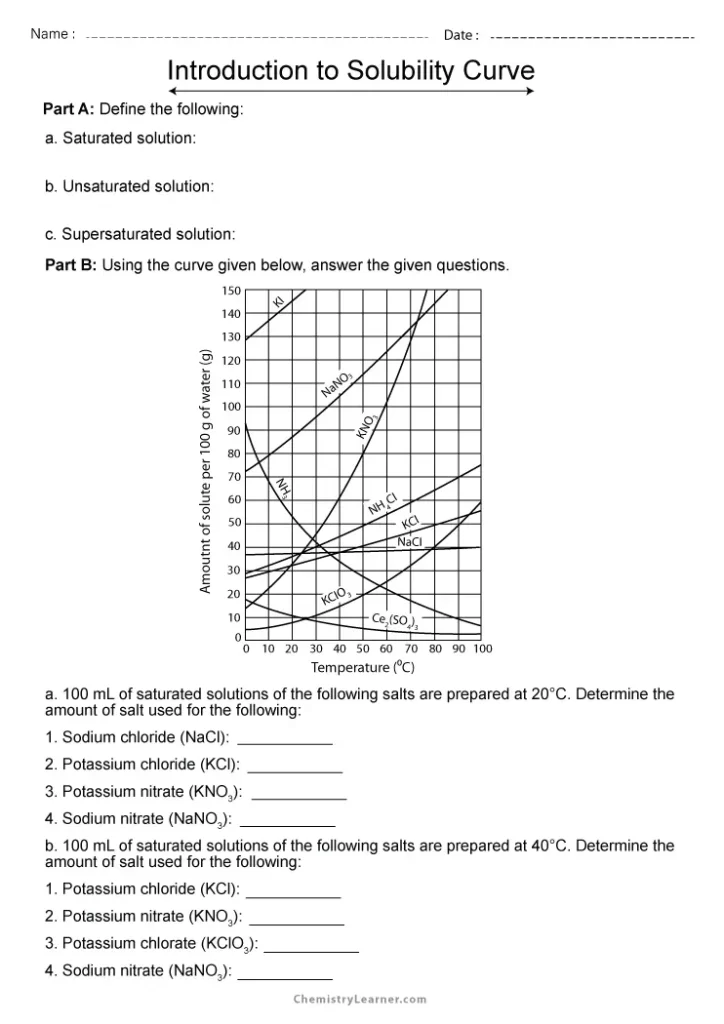
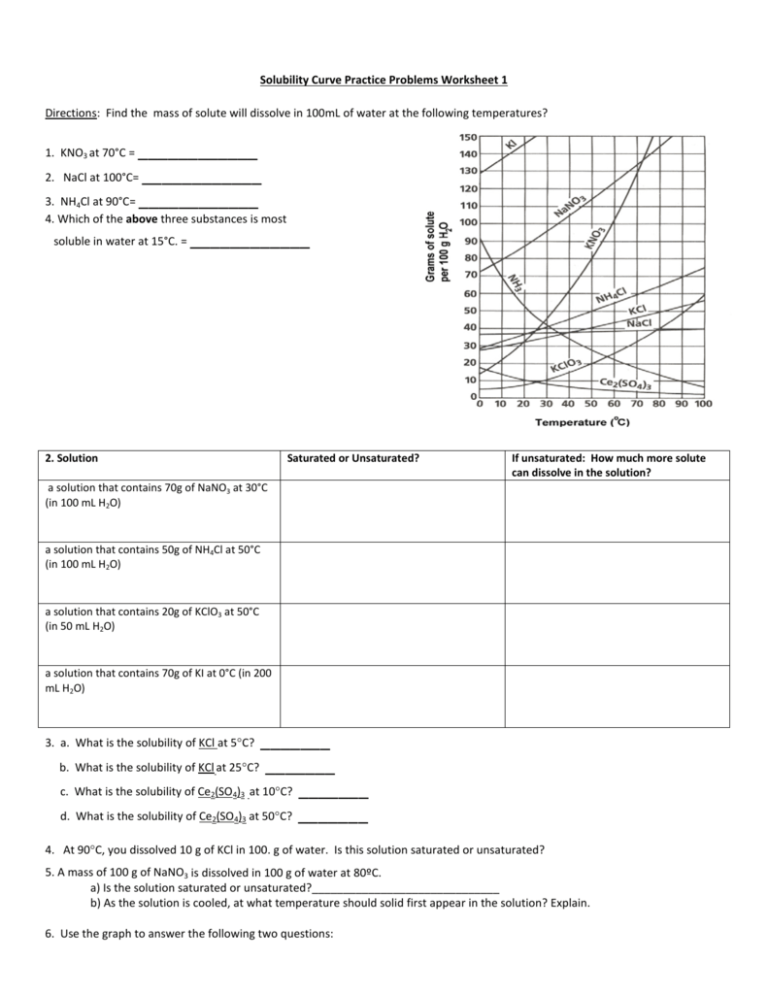
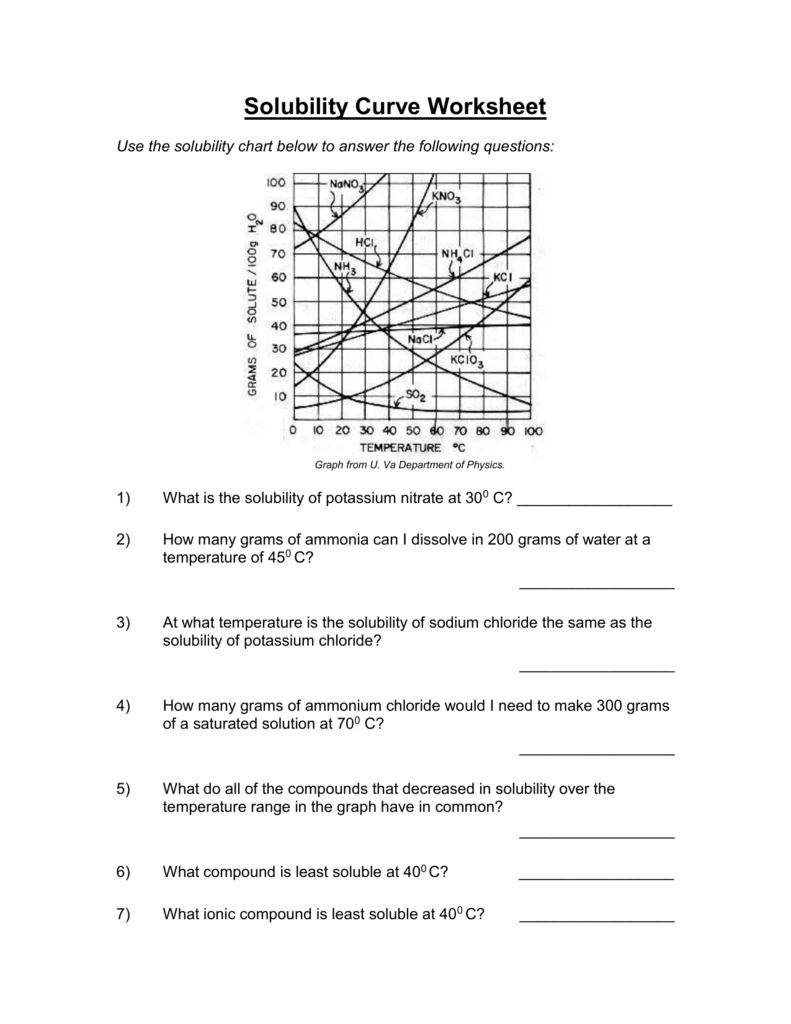
Solubility Curve Worksheet With Answers - If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have prepared numerous free solubility curve. Curve represents the amount of solute which will precipitate out. 100 ml of saturated solutions of the following salts are prepared at. Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. What is the solubility of calcium chloride at 5 c? Use the solubility graph to answer the following:
What is the solubility of calcium chloride at 5 c? Curve represents the amount of solute which will precipitate out. 100 ml of saturated solutions of the following salts are prepared at. Use the solubility graph to answer the following: Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have prepared numerous free solubility curve.
100 ml of saturated solutions of the following salts are prepared at. Use the solubility graph to answer the following: Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. What is the solubility of calcium chloride at 5 c? If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have prepared numerous free solubility curve. Curve represents the amount of solute which will precipitate out.
Free Solubility Curve Worksheet with Answer Keys For 2024 Worksheets
If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have prepared numerous free solubility curve. Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. 100 ml of saturated solutions of the following salts are prepared at. Use the solubility graph to answer the following: What is the.
Solubility Curves & Solutions Review Sheet
Use the solubility graph to answer the following: Curve represents the amount of solute which will precipitate out. Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have prepared numerous free solubility curve. 100 ml of saturated solutions.
SOLUTION Solubility curve worksheet Studypool
100 ml of saturated solutions of the following salts are prepared at. What is the solubility of calcium chloride at 5 c? Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. Curve represents the amount of solute which will precipitate out. Use the solubility graph to answer the following:
Solubility Curve Worksheets Answers
Use the solubility graph to answer the following: Curve represents the amount of solute which will precipitate out. Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. 100 ml of saturated solutions of the following salts are prepared at. If you are struggling with the problems concerning the solubility curve, please don’t be worried,.
Solubility And Solubility Curves
If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have prepared numerous free solubility curve. Use the solubility graph to answer the following: What is the solubility of calcium chloride at 5 c? Curve represents the amount of solute which will precipitate out. Using the table above, determine whether the following solutions would.
Reading Solubility Graphs Worksheet
Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. Use the solubility graph to answer the following: Curve represents the amount of solute which will precipitate out. If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have prepared numerous free solubility curve. What is the solubility of.
Solubility Curves Key Worksheet
100 ml of saturated solutions of the following salts are prepared at. Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have prepared numerous free solubility curve. What is the solubility of calcium chloride at 5 c? Curve.
Free Printable Solubility Curve Worksheets
Curve represents the amount of solute which will precipitate out. Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. What is the solubility of calcium chloride at 5 c? Use the solubility graph to answer the following: If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have.
Solubility Curve Worksheet Key
Use the solubility graph to answer the following: Curve represents the amount of solute which will precipitate out. 100 ml of saturated solutions of the following salts are prepared at. What is the solubility of calcium chloride at 5 c? If you are struggling with the problems concerning the solubility curve, please don’t be worried, we have prepared numerous free.
Reading Solubility Graphs Worksheet
Use the solubility graph to answer the following: Curve represents the amount of solute which will precipitate out. Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated. What is the solubility of calcium chloride at 5 c? 100 ml of saturated solutions of the following salts are prepared at.
If You Are Struggling With The Problems Concerning The Solubility Curve, Please Don’t Be Worried, We Have Prepared Numerous Free Solubility Curve.
Use the solubility graph to answer the following: What is the solubility of calcium chloride at 5 c? 100 ml of saturated solutions of the following salts are prepared at. Using the table above, determine whether the following solutions would be saturated, unsaturated, or supersaturated.