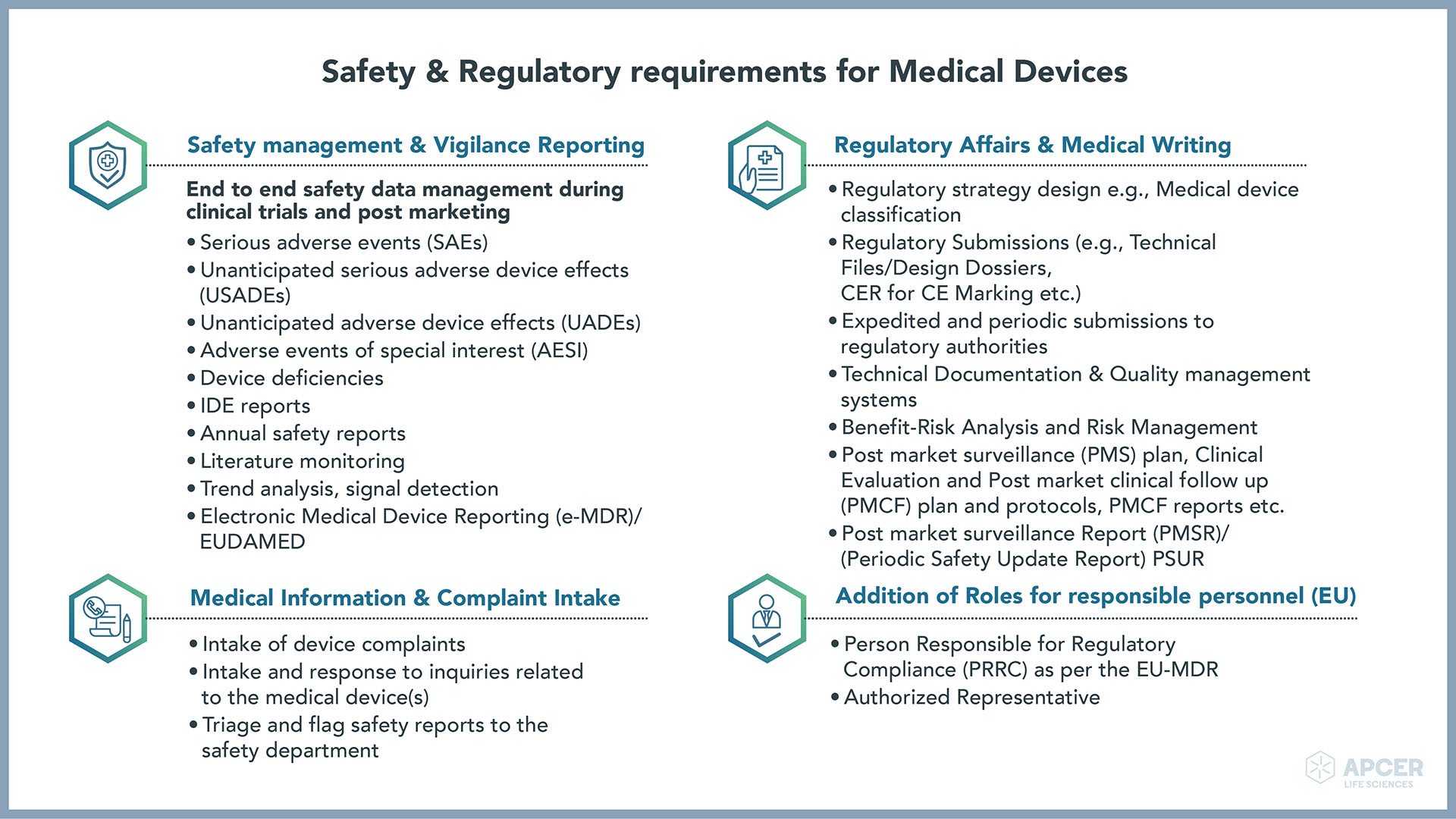
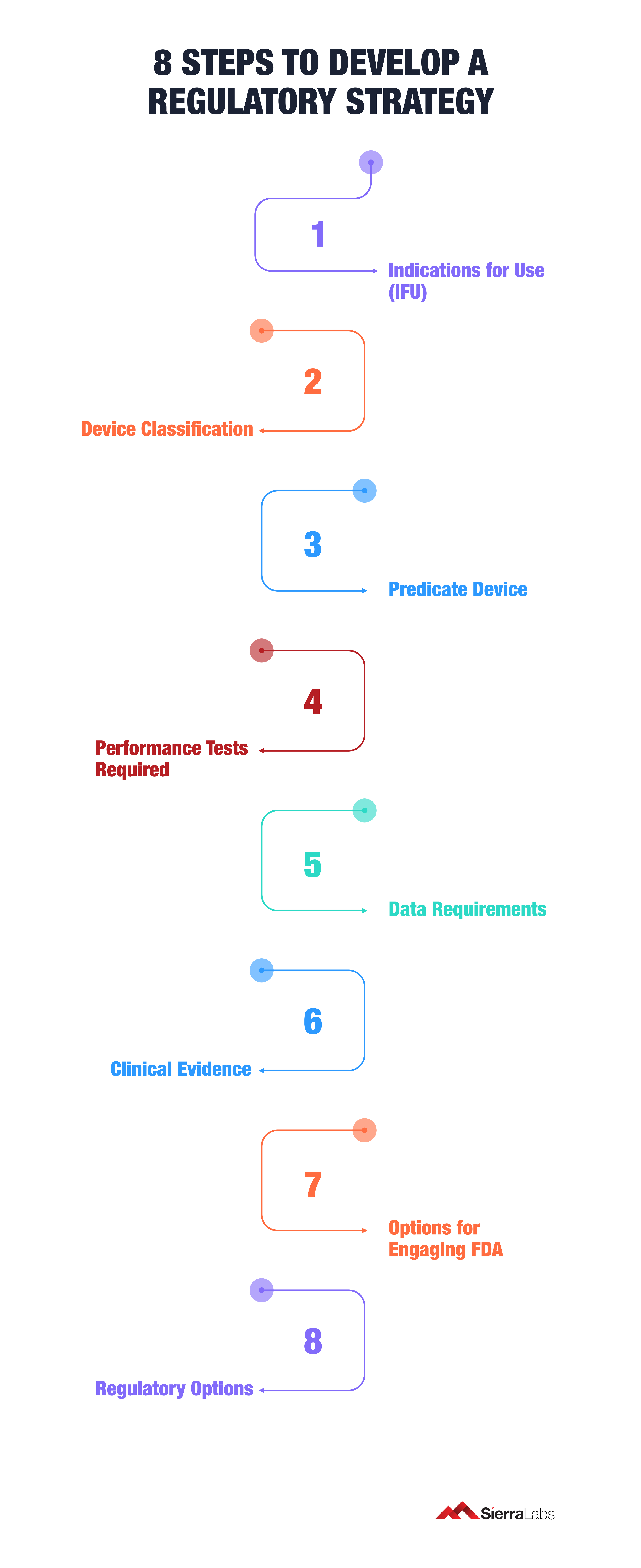
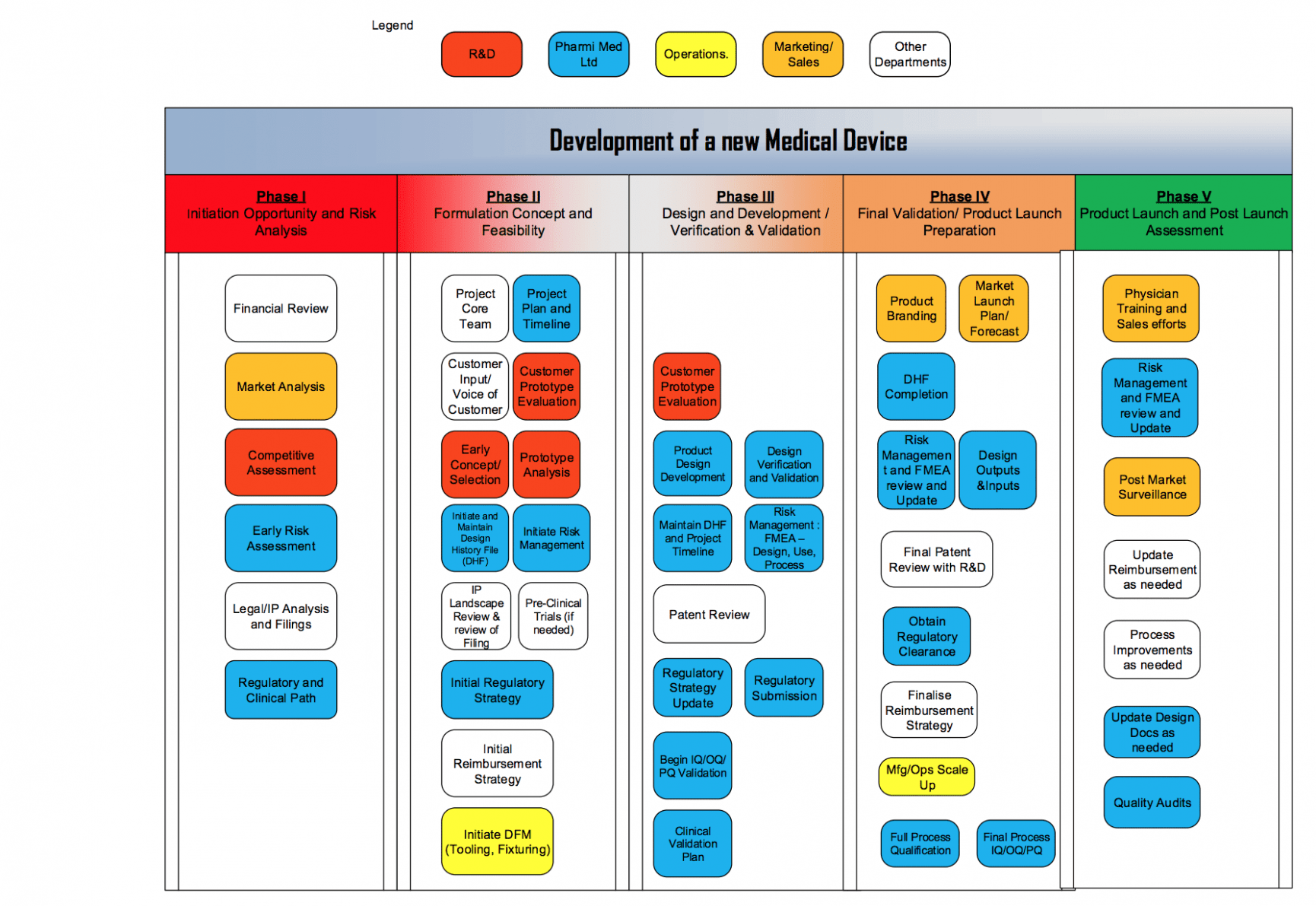
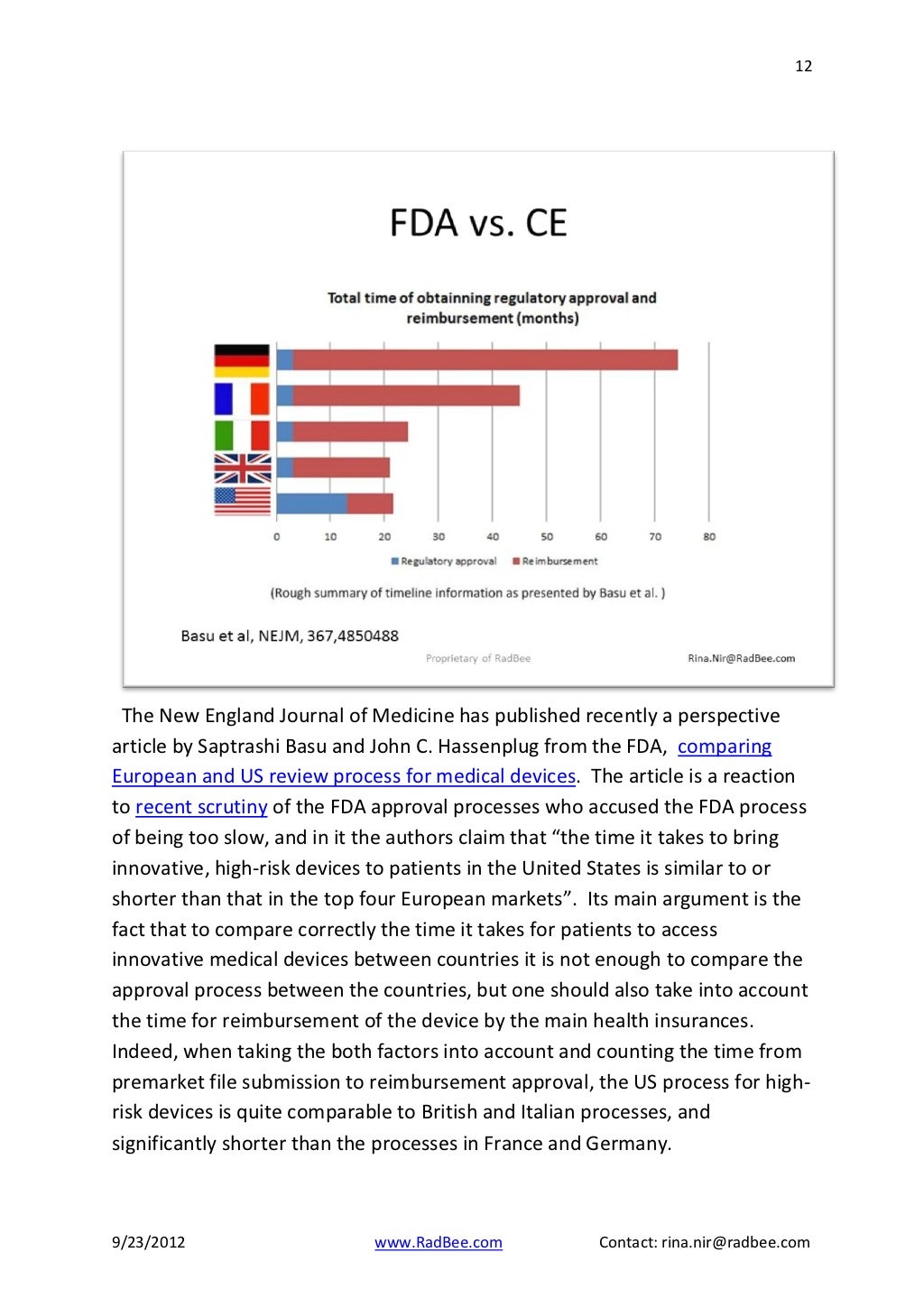
Regulatory Strategy Template For Medical Devices - It is regularly updated pursuant to the. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu. This document lists the applicable standards, norms and regulations for the medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one.
I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu. This document lists the applicable standards, norms and regulations for the medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366. It is regularly updated pursuant to the.
This document lists the applicable standards, norms and regulations for the medical device. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu. It is regularly updated pursuant to the. Covering iso 13485, iec 62304, iso 14971 and iec 62366.
Regulatory Strategy Template For Medical Devices
It is regularly updated pursuant to the. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. Covering iso 13485, iec 62304, iso 14971 and iec 62366..
Regulatory Strategy Template For Medical Devices
This document lists the applicable standards, norms and regulations for the medical device. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. Covering iso 13485, iec 62304, iso 14971 and iec 62366. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical.
Medical Device Regulatory Strategy Template
It is regularly updated pursuant to the. Covering iso 13485, iec 62304, iso 14971 and iec 62366. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu..
Regulatory Strategy Template For Medical Devices
Covering iso 13485, iec 62304, iso 14971 and iec 62366. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu. It is regularly updated pursuant to the. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one..
Medical Device Regulatory Strategy Template
Covering iso 13485, iec 62304, iso 14971 and iec 62366. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. It is regularly updated pursuant to the. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu..
Medical Device Regulatory Strategy Template
Covering iso 13485, iec 62304, iso 14971 and iec 62366. This document lists the applicable standards, norms and regulations for the medical device. It is regularly updated pursuant to the. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. Use this sop regulatory strategy template to define and.
Medical Device Design And Development Plan Template
Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu. This document lists the applicable standards, norms and regulations for the medical device. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. It is regularly updated.
Regulatory Strategy Template For Medical Devices
It is regularly updated pursuant to the. I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. Covering iso 13485, iec 62304, iso 14971 and iec 62366. This document lists the applicable standards, norms and regulations for the medical device. Use this sop regulatory strategy template to define and.
Medical Device Regulatory Strategy Template
I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. It is regularly updated pursuant to the. This document lists the applicable standards, norms and regulations for the medical device. Covering iso 13485, iec 62304, iso 14971 and iec 62366. Use this sop regulatory strategy template to define and.
Regulatory Strategy Template For Medical Devices
I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. This document lists the applicable standards, norms and regulations for the medical device. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu. It is regularly updated.
This Document Lists The Applicable Standards, Norms And Regulations For The Medical Device.
I am looking for templates for us, canada and eu regulatory compliance for medical devices, that would include the elements one. It is regularly updated pursuant to the. Use this sop regulatory strategy template to define and document your organization’s approach for bringing medical devices to the us and eu. Covering iso 13485, iec 62304, iso 14971 and iec 62366.