Process Validation Template - Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential. This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation. The purpose of this process validation protocol is to ensure that the manufacturing process.
The purpose of this process validation protocol is to ensure that the manufacturing process. Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential. This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation.
The purpose of this process validation protocol is to ensure that the manufacturing process. Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential. This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation.
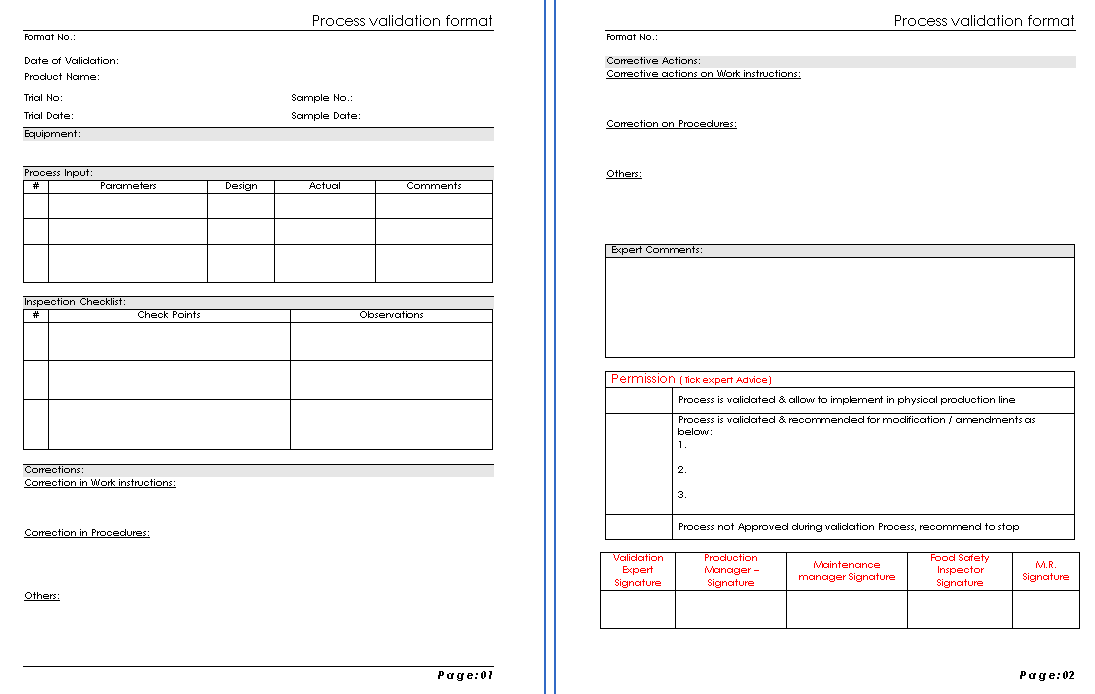
Process validation format
The purpose of this process validation protocol is to ensure that the manufacturing process. This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation. Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential.
Template For Process Validation Protocol PDF Verification And
This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation. The purpose of this process validation protocol is to ensure that the manufacturing process. Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential.
Free Operations Process Validation Form Template Edit Online
This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation. Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential. The purpose of this process validation protocol is to ensure that the manufacturing process.
Free ISO 13485 Process Validation Template
The purpose of this process validation protocol is to ensure that the manufacturing process. Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential. This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation.
Tem290 Process Validation Protocol Template Sample Verification And
Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential. The purpose of this process validation protocol is to ensure that the manufacturing process. This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation.
Process Validation Record format Excel PDF Sample
Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential. The purpose of this process validation protocol is to ensure that the manufacturing process. This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation.
Process Validation Templates at
Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential. This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation. The purpose of this process validation protocol is to ensure that the manufacturing process.
MustHave Process Validation Templates with Samples and Examples
This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation. The purpose of this process validation protocol is to ensure that the manufacturing process. Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential.
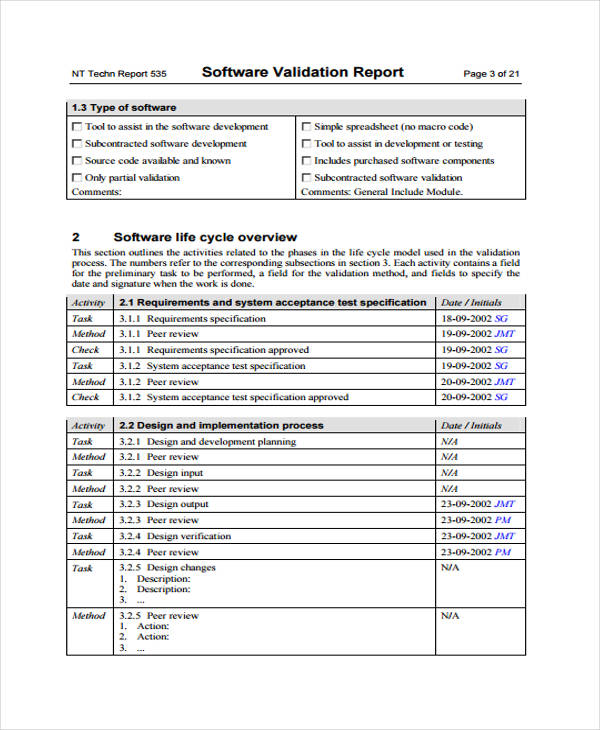
Process Validation Validation Report Template
The purpose of this process validation protocol is to ensure that the manufacturing process. This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation. Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential.
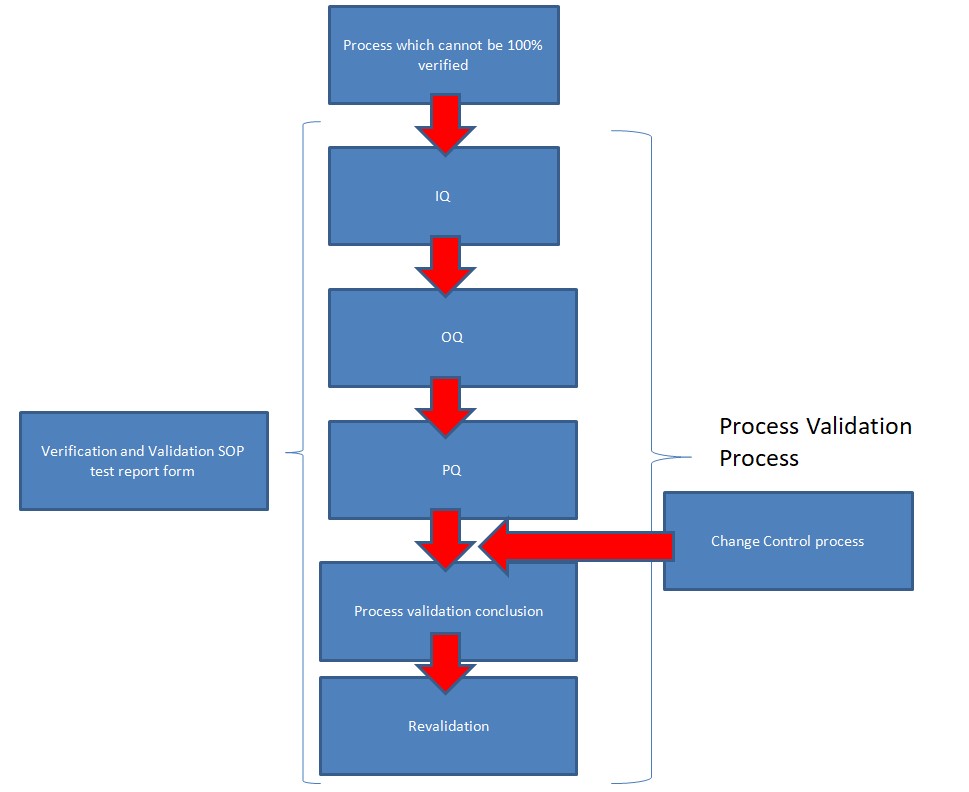
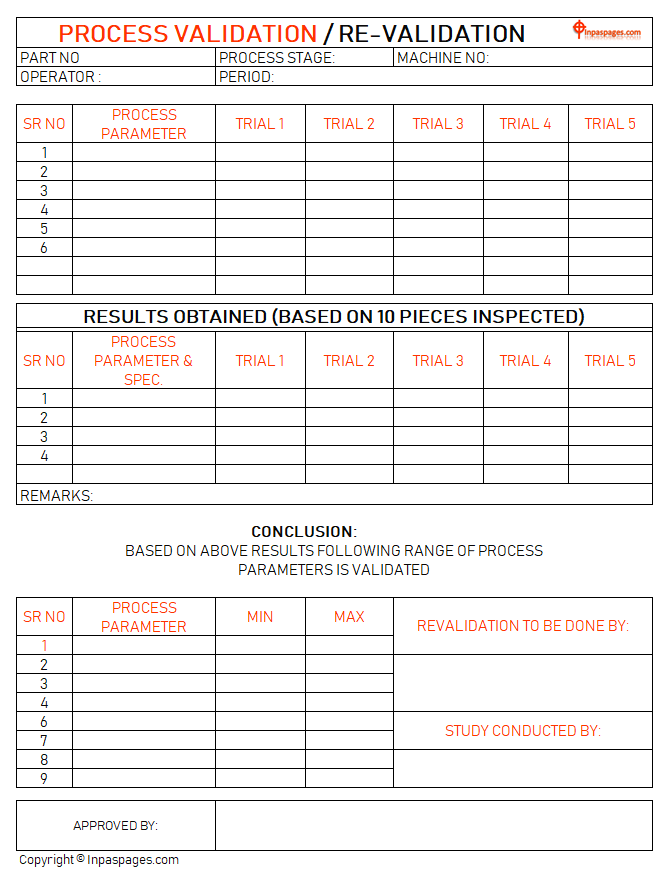
Process validation and revalidation study
This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation. Process(es)subjecttoprocessvalidation mixing filling tableting release private&confidential. The purpose of this process validation protocol is to ensure that the manufacturing process.
Process(Es)Subjecttoprocessvalidation Mixing Filling Tableting Release Private&Confidential.
This process validation report template has been designed to make it easier for validation managers to perform equipment criticality and risk assessment, compare acceptance criteria against performance test results, and specify areas of deviation. The purpose of this process validation protocol is to ensure that the manufacturing process.