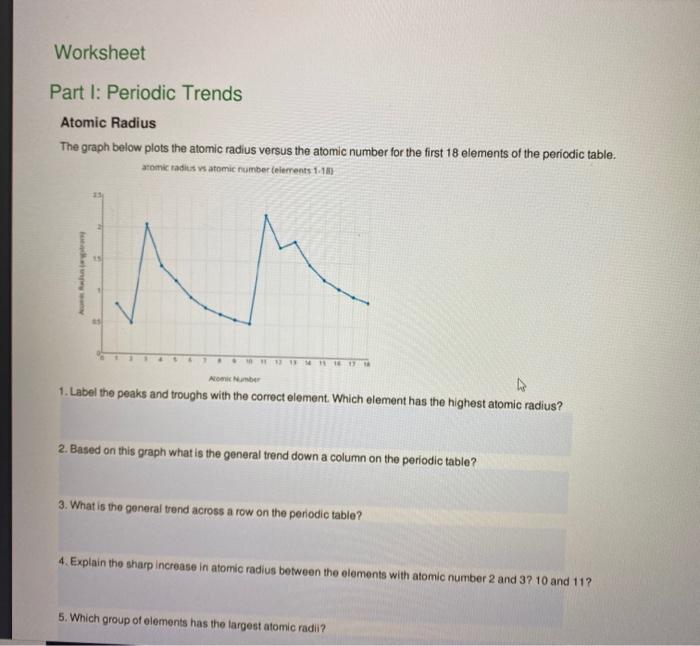
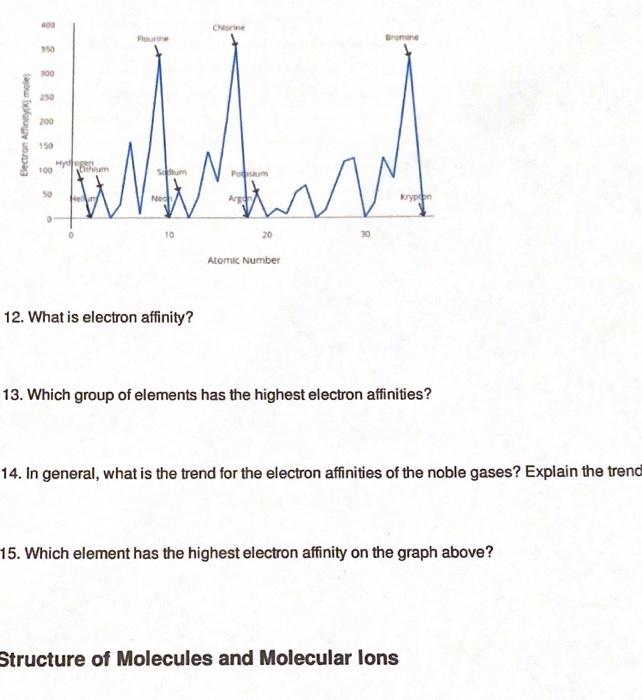
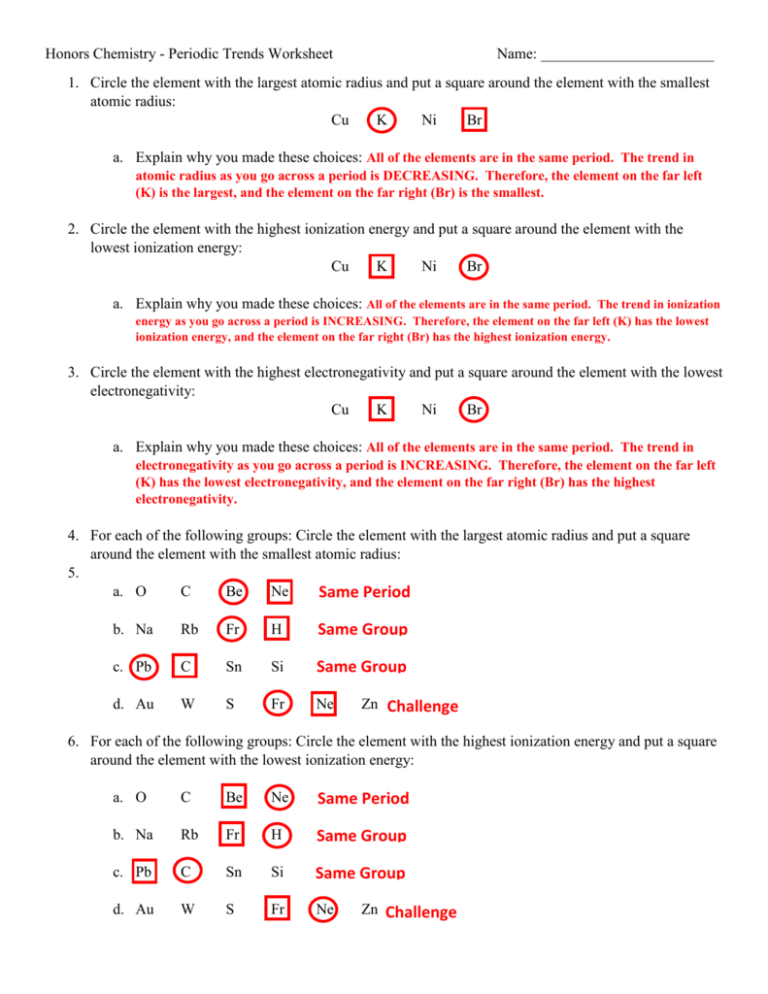
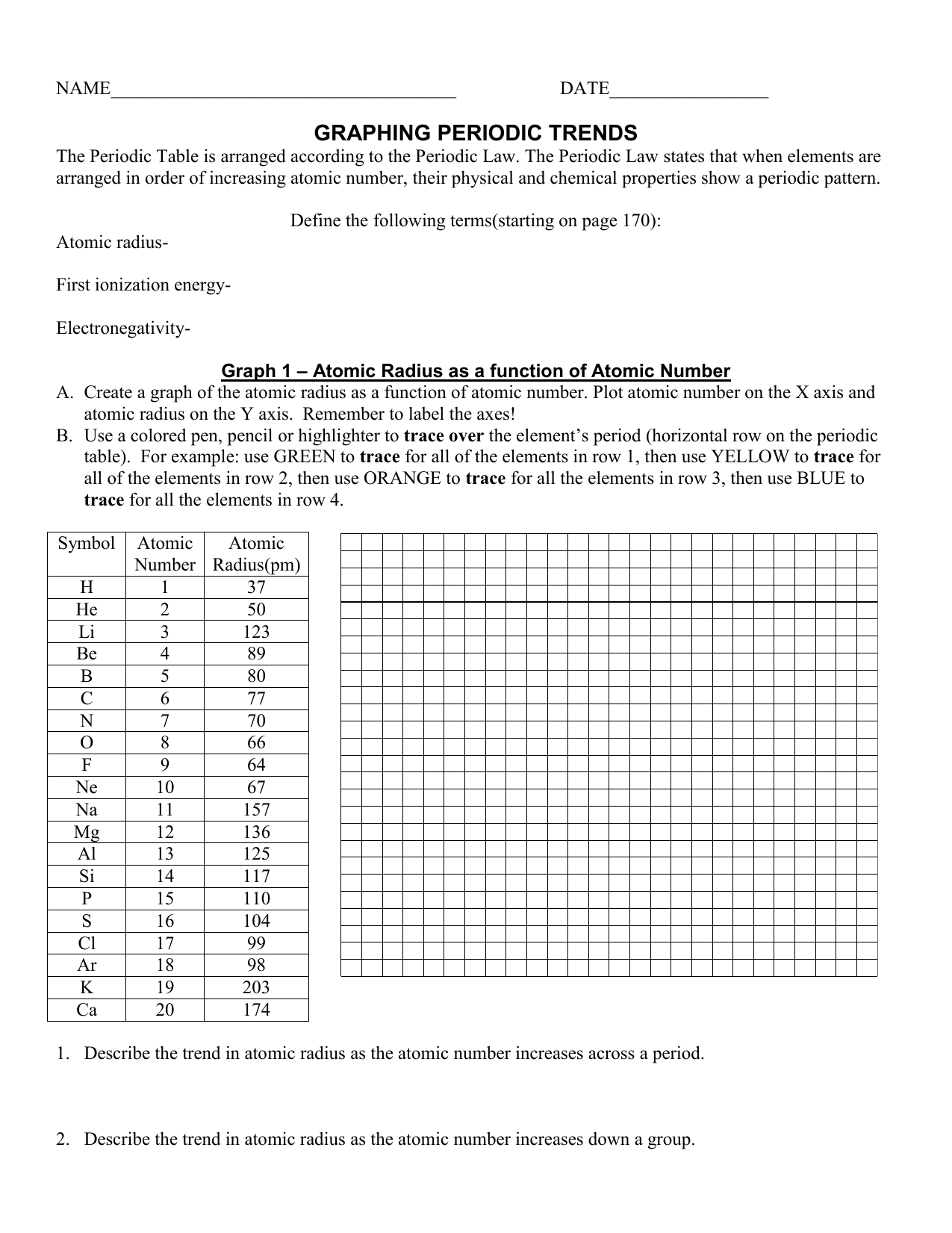
Periodic Trends Atomic Radius Worksheet Answers - For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Which of the following in each pair has the larger atomic radius? Draw arrow showing which directions atomic radius increases. Periodic trends tutorial 2 1. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Use your notes to answer the following questions. Compare the effects of atomic radius on the first ionization energy of an atom.
Which of the following in each pair has the larger atomic radius? 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Periodic trends tutorial 2 1. Compare the effects of atomic radius on the first ionization energy of an atom. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Draw arrow showing which directions atomic radius increases. Use your notes to answer the following questions.
Compare the effects of atomic radius on the first ionization energy of an atom. Periodic trends tutorial 2 1. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Draw arrow showing which directions atomic radius increases. Which of the following in each pair has the larger atomic radius? Use your notes to answer the following questions. For each of the following sets of atoms, rank the atoms from smallest to largest atomic.
Periodic Trends Worksheets With Answers
1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Periodic trends tutorial 2 1. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Which of the following in each pair has the larger atomic radius? Compare the effects of atomic radius on the first ionization energy of.
Solved Worksheet Part I Periodic Trends Atomic Radius The
Use your notes to answer the following questions. Periodic trends tutorial 2 1. Which of the following in each pair has the larger atomic radius? 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Draw arrow showing which directions atomic radius increases.
Solved Worksheet Part I Periodic Trends Atomic Radius The
Use your notes to answer the following questions. Which of the following in each pair has the larger atomic radius? For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Compare the effects of atomic radius on the first ionization energy of an atom. Periodic trends tutorial 2 1.
Periodic Trends Atomic Radius Worksheet Answers Printable Calendars
Draw arrow showing which directions atomic radius increases. Compare the effects of atomic radius on the first ionization energy of an atom. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Use your notes to answer the following.
Graphing Periodic Trends Worksheet Answers Printable Calendars AT A
Periodic trends tutorial 2 1. Draw arrow showing which directions atomic radius increases. Compare the effects of atomic radius on the first ionization energy of an atom. Which of the following in each pair has the larger atomic radius? For each of the following sets of atoms, rank the atoms from smallest to largest atomic.
Periodic Trends Worksheet Atomic Radius Which atom in each pair has
Periodic trends tutorial 2 1. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Compare the effects of atomic radius on the first ionization energy of an atom. Use your notes to answer the following questions. For each of the following sets of atoms, rank the atoms from smallest to largest atomic.
Periodic Trends Atomic Radius Worksheet Answers
1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Compare the effects of atomic radius on the first ionization energy of an atom. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Use your notes to answer the following questions. Periodic trends tutorial 2 1.
Periodic Table Atomic Radius Chem Worksheet 61 2024 Periodic Table
Which of the following in each pair has the larger atomic radius? Draw arrow showing which directions atomic radius increases. Use your notes to answer the following questions. Compare the effects of atomic radius on the first ionization energy of an atom. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___
Periodic Trends Worksheet Answers
Compare the effects of atomic radius on the first ionization energy of an atom. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Which of the following in each pair has the larger atomic radius? 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Periodic trends tutorial.
Periodic Trends Worksheet Answers
For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Use your notes to answer the following questions. Which of the following in each pair has the larger atomic radius? 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Periodic trends tutorial 2 1.
1) Li Or K ___K____ 2) Ca Or Ni ___Ca___ 3) Ga Or B ___Ga___
Draw arrow showing which directions atomic radius increases. Use your notes to answer the following questions. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Periodic trends tutorial 2 1.
Compare The Effects Of Atomic Radius On The First Ionization Energy Of An Atom.
Which of the following in each pair has the larger atomic radius?