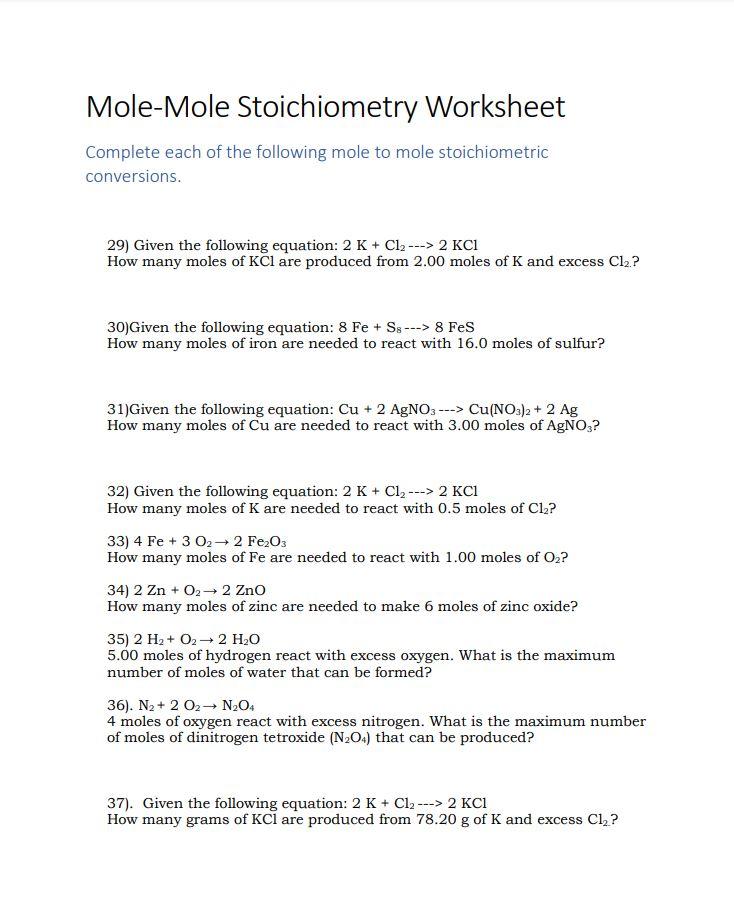
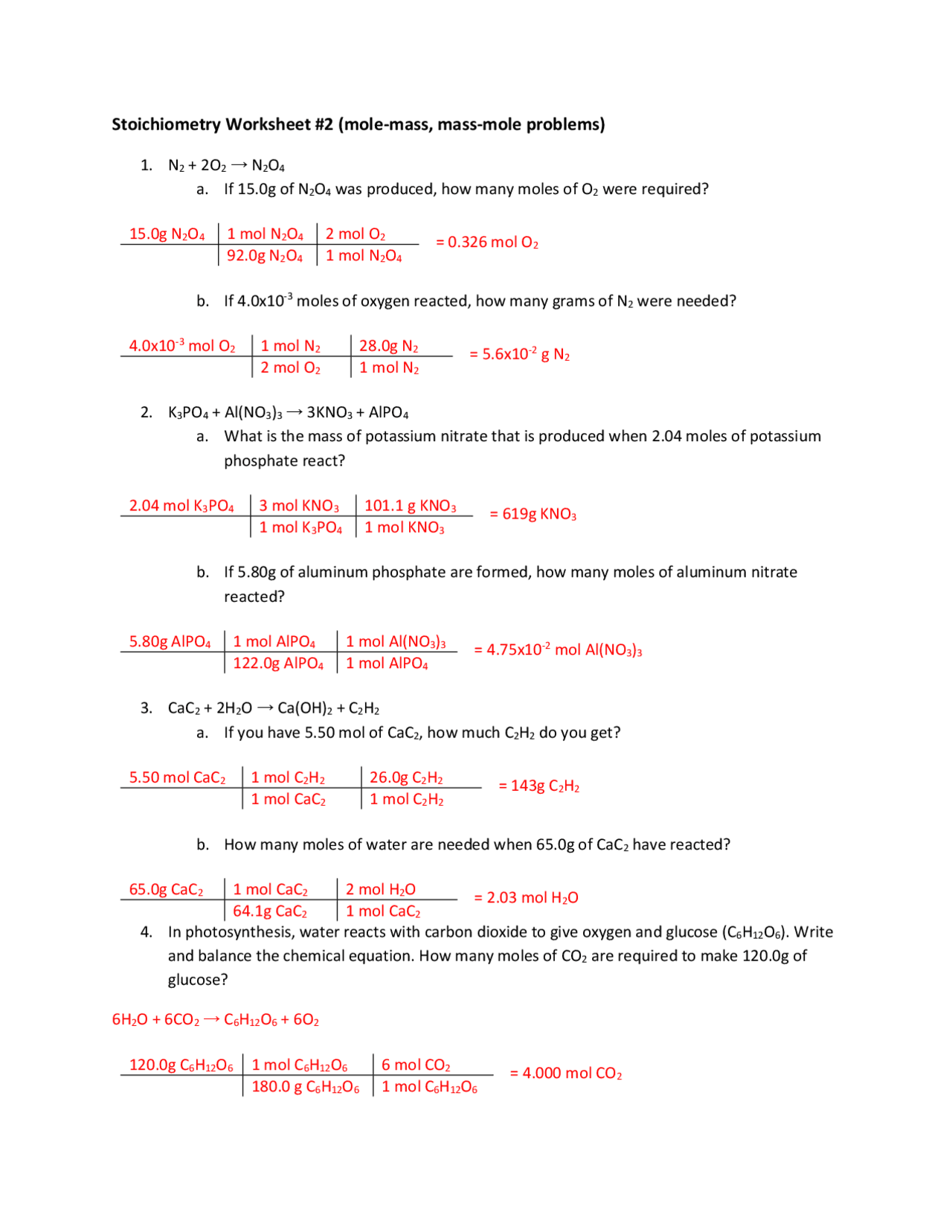
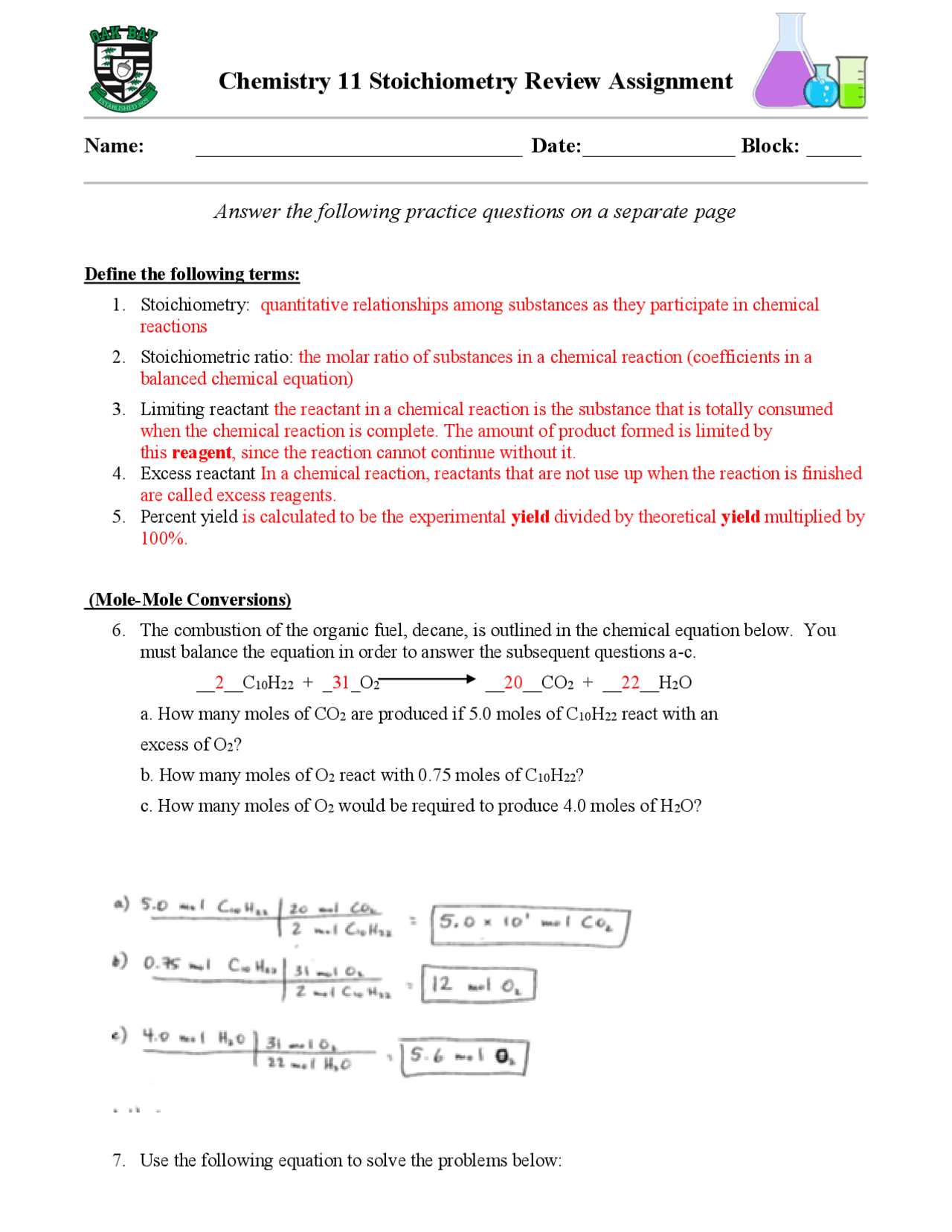
Mole To Mole Stoichiometry Worksheet With Answers - Double lined boxes are conversion factors to convert from one quantity to another. How many moles of cu are needed to react with 3.50 moles of agno3? The atomic weight of carbon is 12.0107 u, so. Grams a x 1 mole a x y mole b x g b.
The atomic weight of carbon is 12.0107 u, so. How many moles of cu are needed to react with 3.50 moles of agno3? Double lined boxes are conversion factors to convert from one quantity to another. Grams a x 1 mole a x y mole b x g b.
The atomic weight of carbon is 12.0107 u, so. Double lined boxes are conversion factors to convert from one quantity to another. How many moles of cu are needed to react with 3.50 moles of agno3? Grams a x 1 mole a x y mole b x g b.
Solved MoleMole Stoichiometry Worksheet Complete each of
How many moles of cu are needed to react with 3.50 moles of agno3? Double lined boxes are conversion factors to convert from one quantity to another. Grams a x 1 mole a x y mole b x g b. The atomic weight of carbon is 12.0107 u, so.
Molarity And Stoichiometry Worksheet
The atomic weight of carbon is 12.0107 u, so. How many moles of cu are needed to react with 3.50 moles of agno3? Double lined boxes are conversion factors to convert from one quantity to another. Grams a x 1 mole a x y mole b x g b.
Molemole Stoichiometry Worksheet Pdf With Answers
Grams a x 1 mole a x y mole b x g b. The atomic weight of carbon is 12.0107 u, so. How many moles of cu are needed to react with 3.50 moles of agno3? Double lined boxes are conversion factors to convert from one quantity to another.
Free mole problems worksheet with answers, Download Free mole problems
The atomic weight of carbon is 12.0107 u, so. Double lined boxes are conversion factors to convert from one quantity to another. How many moles of cu are needed to react with 3.50 moles of agno3? Grams a x 1 mole a x y mole b x g b.
Mole To Mole Stoichiometry Calculations Worksheet
How many moles of cu are needed to react with 3.50 moles of agno3? The atomic weight of carbon is 12.0107 u, so. Double lined boxes are conversion factors to convert from one quantity to another. Grams a x 1 mole a x y mole b x g b.
Worksheet Stoichiometry Mole to Mole Ratio with Answers Exercises
The atomic weight of carbon is 12.0107 u, so. How many moles of cu are needed to react with 3.50 moles of agno3? Grams a x 1 mole a x y mole b x g b. Double lined boxes are conversion factors to convert from one quantity to another.
Unit Stoichiometry Answer key “MoleMole Calculations” WS 1
The atomic weight of carbon is 12.0107 u, so. Double lined boxes are conversion factors to convert from one quantity to another. How many moles of cu are needed to react with 3.50 moles of agno3? Grams a x 1 mole a x y mole b x g b.
Molemole Stoichiometry Worksheet
How many moles of cu are needed to react with 3.50 moles of agno3? The atomic weight of carbon is 12.0107 u, so. Double lined boxes are conversion factors to convert from one quantity to another. Grams a x 1 mole a x y mole b x g b.
Solved Stoichiometry Worksheet 1 MoletoMole and
Grams a x 1 mole a x y mole b x g b. Double lined boxes are conversion factors to convert from one quantity to another. The atomic weight of carbon is 12.0107 u, so. How many moles of cu are needed to react with 3.50 moles of agno3?
The Atomic Weight Of Carbon Is 12.0107 U, So.
How many moles of cu are needed to react with 3.50 moles of agno3? Double lined boxes are conversion factors to convert from one quantity to another. Grams a x 1 mole a x y mole b x g b.