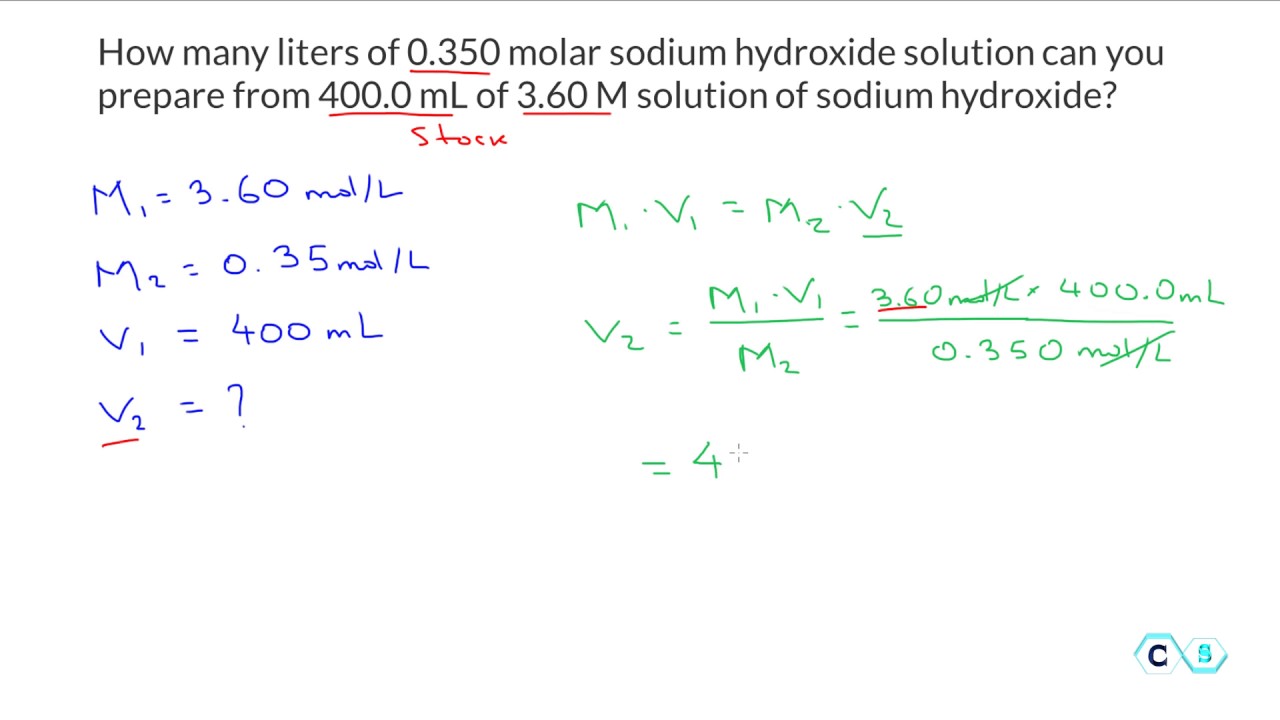
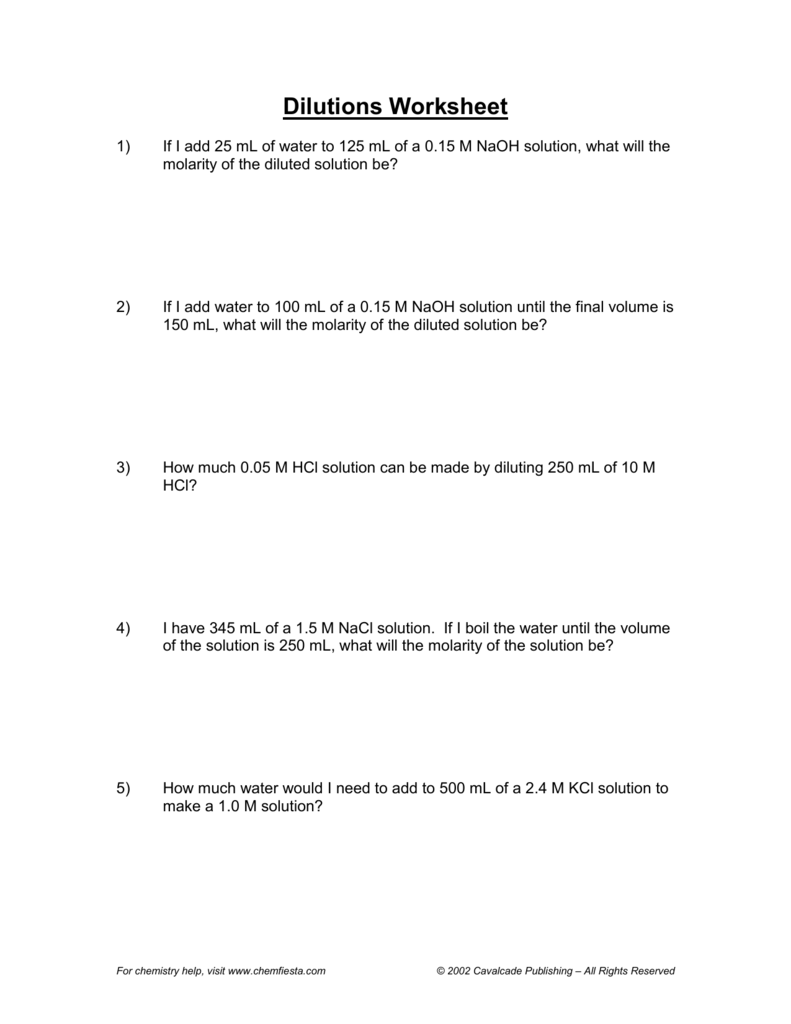
Molarity Dilution Worksheet - 2) how many grams of beryllium. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. What is the molarity of the following solutions given that: 1) for each of the following solutions, the number of moles of solute is given, followed by the total. Molarity and dilution worksheet name: 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution.
What is the molarity of the following solutions given that: 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. 1) for each of the following solutions, the number of moles of solute is given, followed by the total. 2) how many grams of beryllium. Molarity and dilution worksheet name:
1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. 1) for each of the following solutions, the number of moles of solute is given, followed by the total. Molarity and dilution worksheet name: What is the molarity of the following solutions given that: 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. 2) how many grams of beryllium.
Molarity Dilution Worksheet Printable Word Searches
What is the molarity of the following solutions given that: 2) how many grams of beryllium. Molarity and dilution worksheet name: 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.
Molarity and dilution worksheets 1 Molarity Problems Worksheet M = n n= moles V V must
1) for each of the following solutions, the number of moles of solute is given, followed by the total. What is the molarity of the following solutions given that: Molarity and dilution worksheet name: 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. 2) how many grams of beryllium.
Molarity By Dilution Worksheets
1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. What is the molarity of the following solutions given that: 1) for each of the following solutions, the number of moles of solute is given, followed by the total. 2) how many grams of beryllium. 1) 1.0 moles.
Molarity By Dilution Worksheet
1) for each of the following solutions, the number of moles of solute is given, followed by the total. 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. 2) how many grams of beryllium. What is the molarity of the following solutions given that: Molarity and dilution.
Dilutions And Molarity Worksheet Molarity And Dilutions N
1) for each of the following solutions, the number of moles of solute is given, followed by the total. 2) how many grams of beryllium. 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of.
Molarity
What is the molarity of the following solutions given that: 1) for each of the following solutions, the number of moles of solute is given, followed by the total. 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. 2) how many grams of beryllium. Molarity and dilution.
Dilutions And Molarity Worksheet Printable Calendars AT A GLANCE
1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. Molarity and dilution worksheet name: 1) for each of the following solutions, the number of moles of solute is given, followed by the total..
Molarity And Dilution Worksheet Answer Key
1) for each of the following solutions, the number of moles of solute is given, followed by the total. 2) how many grams of beryllium. What is the molarity of the following solutions given that: Molarity and dilution worksheet name: 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.
Molarity And Dilution Worksheet kidsworksheetfun
2) how many grams of beryllium. Molarity and dilution worksheet name: 1) for each of the following solutions, the number of moles of solute is given, followed by the total. 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are.
Solved Molarity and Dilutions Tutorial Worksheet Part 1
1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. What is the molarity of the following solutions given that: Molarity and dilution worksheet name: 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution. 1) for each of the following solutions, the number.
Molarity And Dilution Worksheet Name:
2) how many grams of beryllium. 1) what is the molarity of a solution in which 0.45 grams of sodium nitrate are dissolved in 265 ml of solution. What is the molarity of the following solutions given that: 1) 1.0 moles of potassium fluoride is dissolved to make 0.10 l of solution.