Merck Clinical Trial Recruitment Strategies Recent - Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development.
Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial.
Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening.
Velocity Collaborates With Merck to Improve Diversity in Clinical
Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Pharmaceutical companies have.
10 Strategies for Clinical Trials Recruitment Marketing.pdf
The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and.
Global Clinical Trial Operations Merck Careers
Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. We’re grateful to the thousands.
Merck Keynote Clinical Trial Branding by Melissa Sieja at
Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. We’re grateful to the thousands.
5 Proven Patient Recruitment Strategies in Clinical Trials
Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Awareness of challenges and reviewing strategies that can optimise recruitment and retention will facilitate drug development. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. We’re grateful to the thousands.
Patient Recruitment for Clinical Trials Key Challenges, Optimization
The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and.
10 Strategies for Clinical Trials Recruitment Marketing.pdf
Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective. Pharmaceutical companies have.
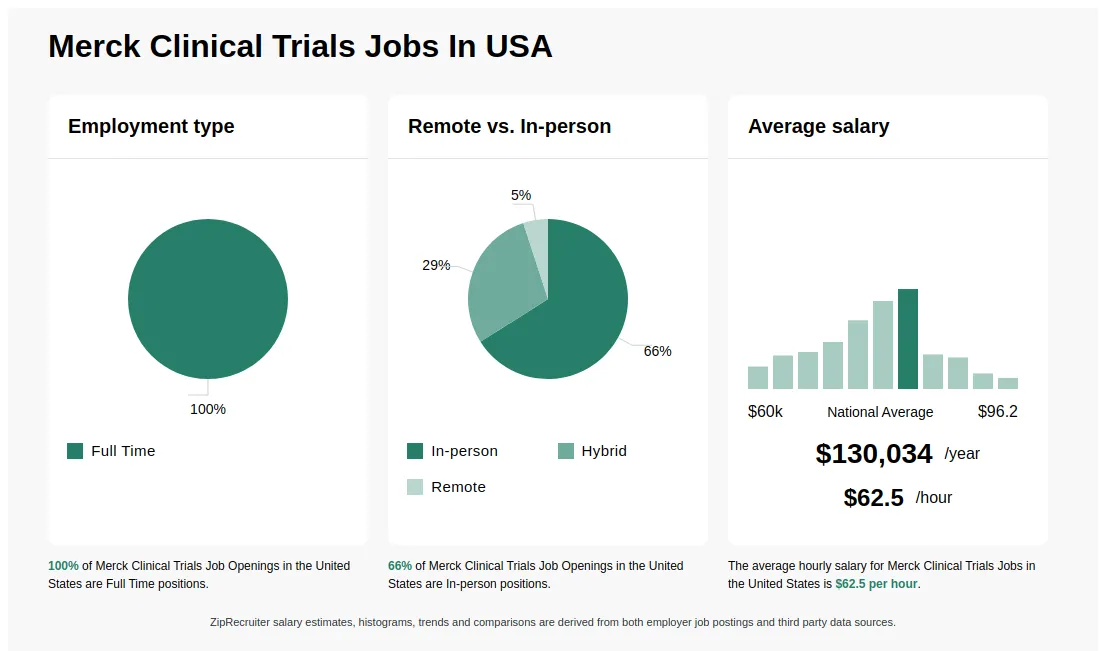
5083/hr Merck Clinical Trials Jobs (NOW HIRING) Feb 2025
We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe.
10 Strategies for Clinical Trials Recruitment Marketing.pdf
The ability to move from intentions to actions in support of diversity, equity, and inclusion in clinical research is vital to opening. Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and.
10 Clinical Trial Recruitment Strategies That Work
Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. The ability to move from intentions to actions.
Awareness Of Challenges And Reviewing Strategies That Can Optimise Recruitment And Retention Will Facilitate Drug Development.
Pharmaceutical companies have an opportunity to drive timelier recruitment by working with patients to develop clinical trial. Recently, four clinical research managers (crm) from merck (known as msd outside the united states and canada). We’re grateful to the thousands of volunteers who participate in our clinical trials — making this all possible. Clinical trials require four phases, in addition to government review and approval, to ensure the treatment being studied is safe and effective.