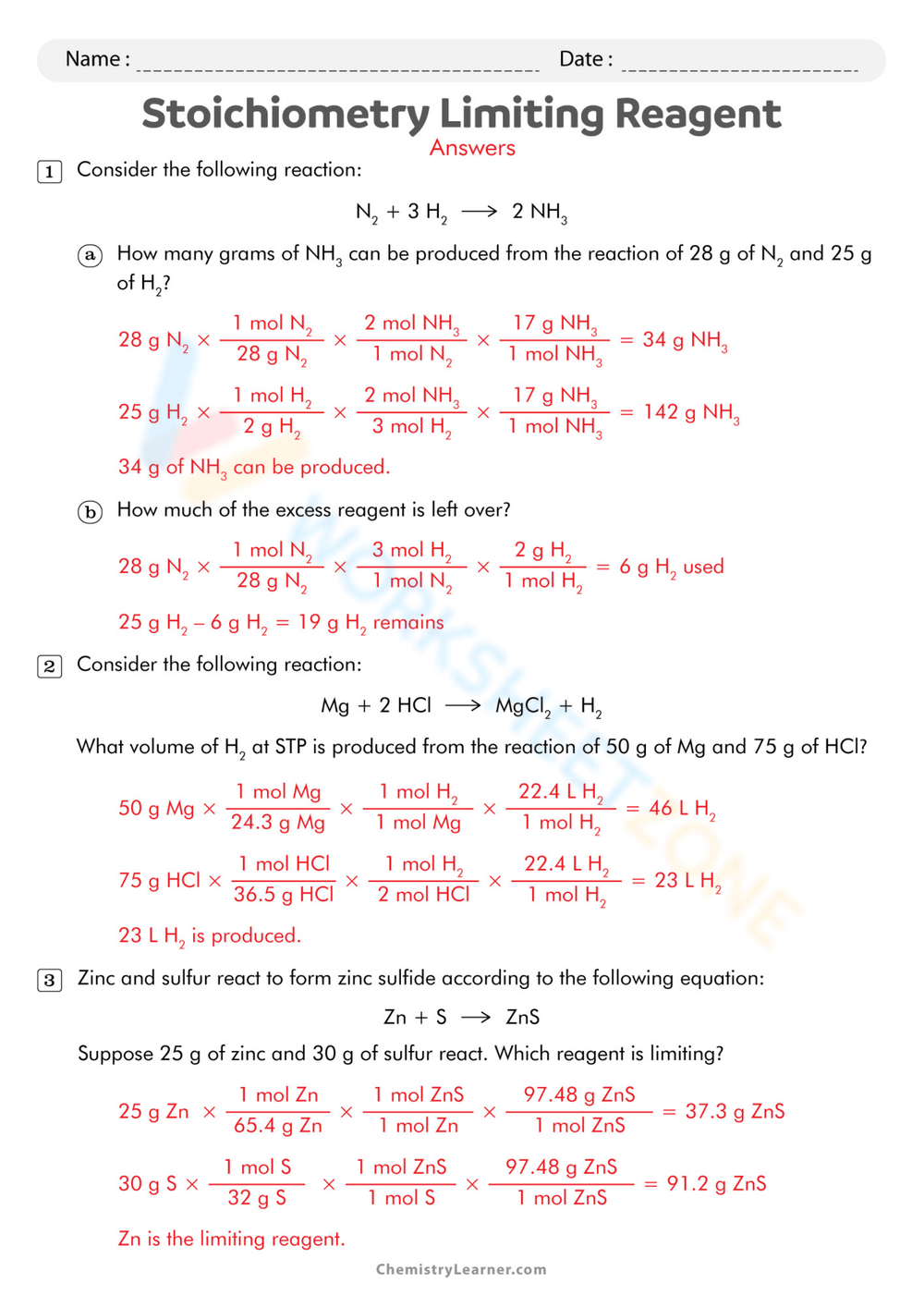
Limiting Reagent Worksheet 2 Answers - What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? How many moles of nh3 can be produced from the reaction of 28 g of n2 ? 3) what is the limiting reagent in the reaction described in problem 2? A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5 m ca (oh)2 solution, determine the limiting reagent. Because sodium iodide is the reagent that causes 8.51 grams of sodium.
3) what is the limiting reagent in the reaction described in problem 2? Because sodium iodide is the reagent that causes 8.51 grams of sodium. What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? How many moles of nh3 can be produced from the reaction of 28 g of n2 ? A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5 m ca (oh)2 solution, determine the limiting reagent.
What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5 m ca (oh)2 solution, determine the limiting reagent. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? 3) what is the limiting reagent in the reaction described in problem 2? Because sodium iodide is the reagent that causes 8.51 grams of sodium.
SOLUTION Moles limiting reagent and molarity worksheet with answers
Because sodium iodide is the reagent that causes 8.51 grams of sodium. 3) what is the limiting reagent in the reaction described in problem 2? How many moles of nh3 can be produced from the reaction of 28 g of n2 ? A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5.
Limiting Reagents Worksheets
3) what is the limiting reagent in the reaction described in problem 2? A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5 m ca (oh)2 solution, determine the limiting reagent. What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0.
Mastering Limiting Reagent Worksheet 2 Answers and Techniques Revealed
What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5 m ca (oh)2 solution, determine the limiting reagent. Because sodium iodide is the reagent that causes 8.51 grams.
Stoichiometry Limiting Reagent Worksheet Worksheets Library
3) what is the limiting reagent in the reaction described in problem 2? Because sodium iodide is the reagent that causes 8.51 grams of sodium. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide.
Limiting Reagent Worksheet 2 Answers
What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? Because sodium iodide is the reagent that causes 8.51 grams of sodium. 3) what is the limiting reagent in the reaction described in problem 2? A) if 40 ml of a 1.0 m hclo4 solution is.
Limiting Reagent Worksheet Worksheets Library
What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? 3) what is the limiting reagent in the reaction described in problem 2? A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5 m ca (oh)2 solution, determine.
Limiting Reagent Worksheet 1 Worksheets Library
A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5 m ca (oh)2 solution, determine the limiting reagent. What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? How many moles of nh3 can be produced from the.
Limiting Reactants Worksheet GCSE Chemistry Beyond Worksheets Library
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? 3) what is the limiting reagent in the reaction described in problem 2? What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? A) if 40 ml of a.
Limiting Reagent Practice Problems Worksheet for 10th Higher Ed
Because sodium iodide is the reagent that causes 8.51 grams of sodium. 3) what is the limiting reagent in the reaction described in problem 2? What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? A) if 40 ml of a 1.0 m hclo4 solution is.
Limiting Reagents 2 Chemsheets Answers
Because sodium iodide is the reagent that causes 8.51 grams of sodium. What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water? A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5 m ca (oh)2 solution, determine the.
How Many Moles Of Nh3 Can Be Produced From The Reaction Of 28 G Of N2 ?
A) if 40 ml of a 1.0 m hclo4 solution is reacted with 60 ml of a 1.5 m ca (oh)2 solution, determine the limiting reagent. Because sodium iodide is the reagent that causes 8.51 grams of sodium. 3) what is the limiting reagent in the reaction described in problem 2? What mass of calcium hydroxide is formed when 10.0 g of cao + h2o → ca(oh)2 calcium oxide reacts with 10.0 g of water?