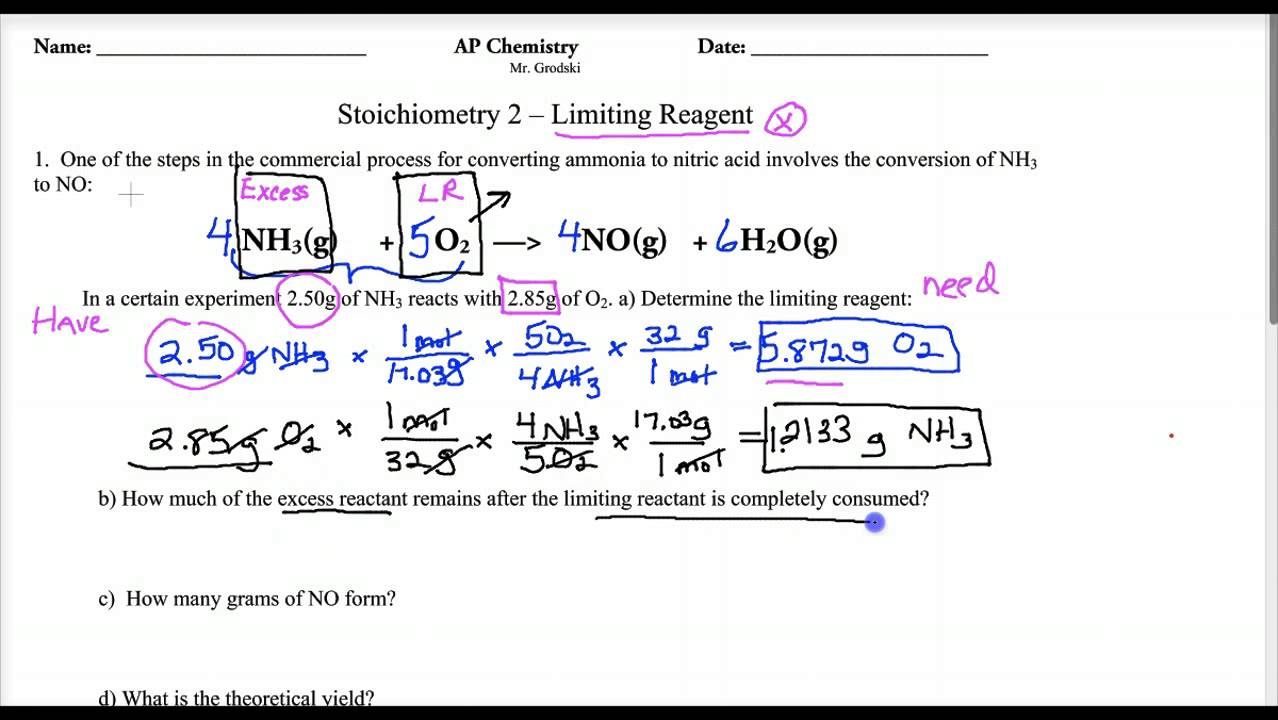
Limiting Reagent Worksheet 1 Answers - Because sodium iodide is the reagent that causes 8.51 grams of sodium. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reagent worksheet #1 1. Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. What is the limiting reagent in the reaction described in problem 2?
Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Limiting reagent worksheet #1 1. Because sodium iodide is the reagent that causes 8.51 grams of sodium. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? What is the limiting reagent in the reaction described in problem 2?
Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Because sodium iodide is the reagent that causes 8.51 grams of sodium. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reagent worksheet #1 1. What is the limiting reagent in the reaction described in problem 2?
SOLUTION Limiting reagent worksheet 1 Studypool
Because sodium iodide is the reagent that causes 8.51 grams of sodium. Limiting reagent worksheet #1 1. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? What is the limiting reagent in the reaction described in problem 2? Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii).
Limiting Reagent Worksheet 1 Worksheets Library
What is the limiting reagent in the reaction described in problem 2? Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Limiting reagent worksheet #1 1. Because sodium iodide is the reagent that causes 8.51 grams of sodium. How many moles of nh3 can be produced from the reaction.
Limiting Reactants Examples With Answers
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Because sodium iodide is the reagent that causes 8.51 grams of sodium. Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. What is the limiting reagent in the reaction described in problem.
Limiting Reagent Worksheet 1 Answers
What is the limiting reagent in the reaction described in problem 2? Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reagent worksheet #1 1. Because sodium iodide is the reagent.
Limiting Reagent Worksheet 1 Answers Printable Word Searches
What is the limiting reagent in the reaction described in problem 2? Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Because sodium iodide is the reagent that causes 8.51 grams of sodium. How many moles of nh3 can be produced from the reaction of 28 g of n2.
Limiting Reagent WS 1 worksheet Live Worksheets
Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Limiting reagent worksheet #1 1. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Because sodium iodide is the reagent that causes 8.51 grams of sodium. What is the limiting reagent in.
How to Find Limiting Reagent Worksheet 1 Answers A StepbyStep Guide
Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Because sodium iodide is the reagent that causes 8.51 grams of sodium. What is the limiting reagent in the reaction described in problem.
Limiting Reagent Worksheets 1 Answers
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Because sodium iodide is the reagent that causes 8.51 grams of sodium. Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Limiting reagent worksheet #1 1. What is the limiting reagent in.
Limiting Reagent Worksheet 1
What is the limiting reagent in the reaction described in problem 2? Limiting reagent worksheet #1 1. How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Because sodium iodide is the reagent.
Limiting Reagent Worksheet 1 Pdf —
How many moles of nh3 can be produced from the reaction of 28 g of n2 ? What is the limiting reagent in the reaction described in problem 2? Because sodium iodide is the reagent that causes 8.51 grams of sodium. Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are.
Because Sodium Iodide Is The Reagent That Causes 8.51 Grams Of Sodium.
Limiting reagent worksheet #1 1. What is the limiting reagent in the reaction described in problem 2? How many moles of nh3 can be produced from the reaction of 28 g of n2 ? Limiting reagent worksheet 1) when copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed.