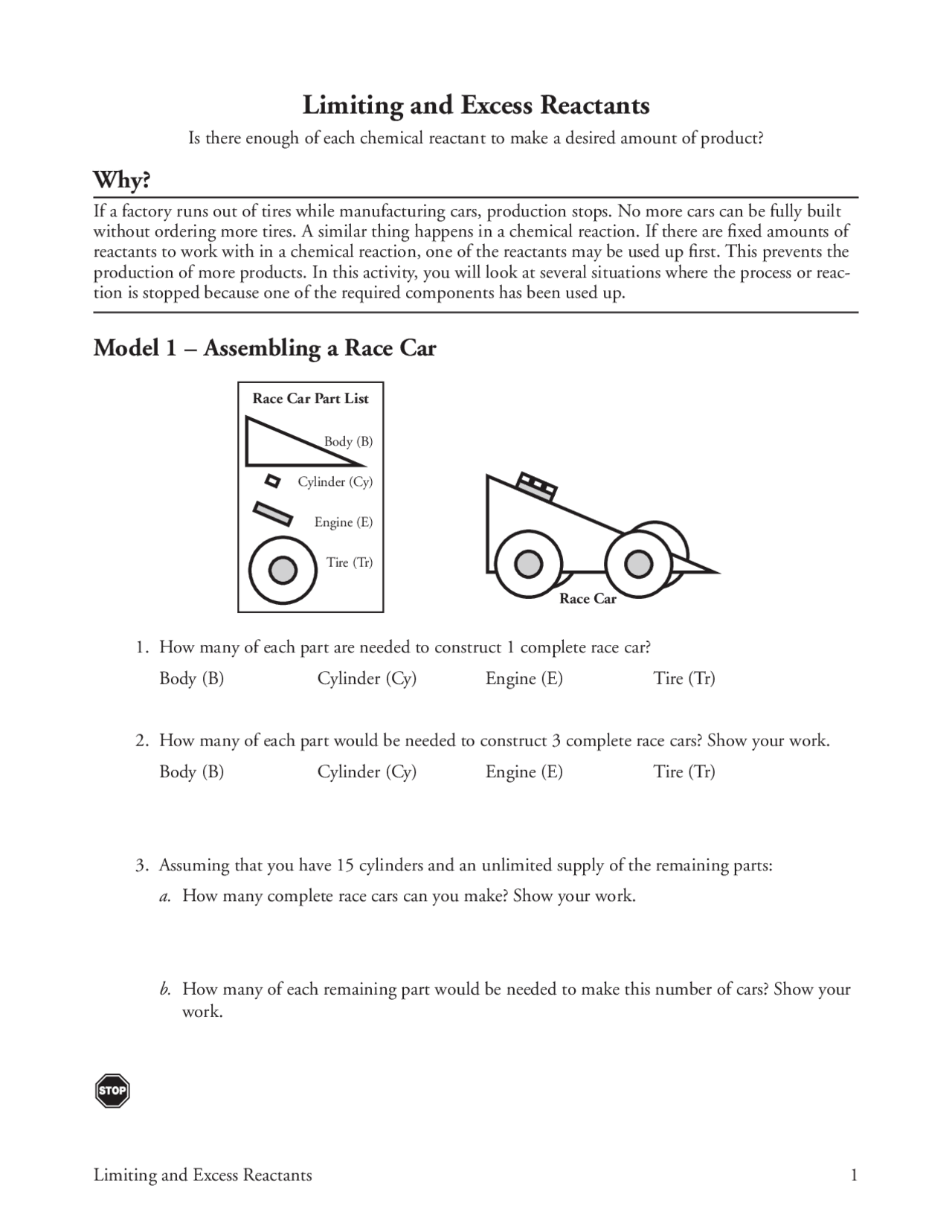
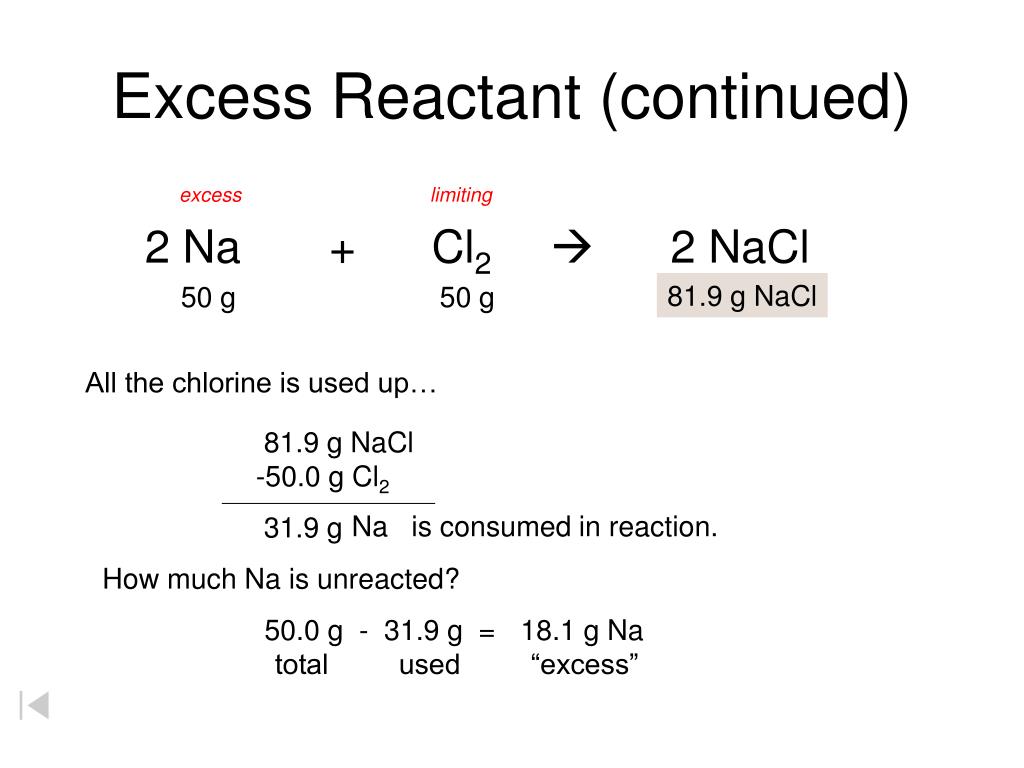
Limiting And Excess Reactants Worksheet With Answers - (a) what is the limiting reactant? Limiting reactant worksheet #1 1. Magnesium is limiting and oxygen is in excess 2. Ch4 + 2 h2o ! How many moles of nh. (b) how many moles of ammonia will form? N 2 + h 2 nh 3 1. Worksheet on excess and limiting reactants/reagents. 4 h2(g) + co2(g) how many liters of hydrogen can be produced from the reaction of. 2 al + 6 hbr → 2 albr3 + 3 h2 a.
Limiting reactant worksheet #1 1. (b) how many moles of ammonia will form? 2 al + 6 hbr → 2 albr3 + 3 h2 a. 4 h2(g) + co2(g) how many liters of hydrogen can be produced from the reaction of. When dealing with questions where the mol of more than one reactant is. (a) what is the limiting reactant? Magnesium is limiting and oxygen is in excess 2. How many moles of nh. Ch4 + 2 h2o ! A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles.
A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles. (a) what is the limiting reactant? Worksheet on excess and limiting reactants/reagents. Limiting reactant worksheet #1 1. Magnesium is limiting and oxygen is in excess 2. 4 h2(g) + co2(g) how many liters of hydrogen can be produced from the reaction of. 2 al + 6 hbr → 2 albr3 + 3 h2 a. Ch4 + 2 h2o ! N 2 + h 2 nh 3 1. When dealing with questions where the mol of more than one reactant is.
24 Limiting and Excess ReactantsS Exercises Chemistry Docsity
Limiting reactant worksheet #1 1. Worksheet on excess and limiting reactants/reagents. 4 h2(g) + co2(g) how many liters of hydrogen can be produced from the reaction of. A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles. N 2 + h 2 nh 3 1.
Limiting And Excess Reactants Worksheet With Answers
2 al + 6 hbr → 2 albr3 + 3 h2 a. Ch4 + 2 h2o ! (b) how many moles of ammonia will form? A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles. 4 h2(g) + co2(g) how many liters of hydrogen can be produced from the reaction of.
Limiting And Excess Reactants Worksheet With Answers
2 al + 6 hbr → 2 albr3 + 3 h2 a. N 2 + h 2 nh 3 1. How many moles of nh. Magnesium is limiting and oxygen is in excess 2. (a) what is the limiting reactant?
Limiting Reagents Worksheets
2 al + 6 hbr → 2 albr3 + 3 h2 a. A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles. 4 h2(g) + co2(g) how many liters of hydrogen can be produced from the reaction of. N 2 + h 2 nh 3 1. Worksheet on excess and limiting reactants/reagents.
14 Limiting and Excess Reactants With Answer Key PDF Ammonia
A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles. Ch4 + 2 h2o ! (a) what is the limiting reactant? Magnesium is limiting and oxygen is in excess 2. 2 al + 6 hbr → 2 albr3 + 3 h2 a.
Limiting And Excess Reactants Ppt
(a) what is the limiting reactant? Limiting reactant worksheet #1 1. Worksheet on excess and limiting reactants/reagents. A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles. 4 h2(g) + co2(g) how many liters of hydrogen can be produced from the reaction of.
Limexcess reagants wksht and answers Practice Problems Limiting
How many moles of nh. (a) what is the limiting reactant? N 2 + h 2 nh 3 1. Ch4 + 2 h2o ! 2 al + 6 hbr → 2 albr3 + 3 h2 a.
Mastering Limiting and Excess Reactants Your Complete Worksheet with
Ch4 + 2 h2o ! When dealing with questions where the mol of more than one reactant is. 4 h2(g) + co2(g) how many liters of hydrogen can be produced from the reaction of. Limiting reactant worksheet #1 1. A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles.
SOLUTION Worksheet with answers limiting reactant and excess reactant
Limiting reactant worksheet #1 1. How many moles of nh. (b) how many moles of ammonia will form? When dealing with questions where the mol of more than one reactant is. A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles.
How Many Moles Of Nh.
A welder has 20.0 moles of acetylene gas (c 2h 2) and 10.0 moles. (b) how many moles of ammonia will form? Magnesium is limiting and oxygen is in excess 2. (a) what is the limiting reactant?
2 Al + 6 Hbr → 2 Albr3 + 3 H2 A.
N 2 + h 2 nh 3 1. Worksheet on excess and limiting reactants/reagents. When dealing with questions where the mol of more than one reactant is. Limiting reactant worksheet #1 1.
Ch4 + 2 H2O !
4 h2(g) + co2(g) how many liters of hydrogen can be produced from the reaction of.