Iso 14971 2019 Risk Management Plan Template - Here are all our posts. The risk management report contains the output and summary of risk. The following policy establishes criteria for risk acceptability following iso. The risk management report template should be modified to suit your company qms and. Iso 14971 is the standard for risk management of medical device software.
Iso 14971 is the standard for risk management of medical device software. The risk management report contains the output and summary of risk. The risk management report template should be modified to suit your company qms and. Here are all our posts. The following policy establishes criteria for risk acceptability following iso.
Iso 14971 is the standard for risk management of medical device software. The following policy establishes criteria for risk acceptability following iso. Here are all our posts. The risk management report template should be modified to suit your company qms and. The risk management report contains the output and summary of risk.
Risk Management for Medical Devices ISO 149712019 Kvalito
The risk management report contains the output and summary of risk. The following policy establishes criteria for risk acceptability following iso. Here are all our posts. The risk management report template should be modified to suit your company qms and. Iso 14971 is the standard for risk management of medical device software.
Creating a Medical Device Risk Management Plan and Doing Analysis
Here are all our posts. Iso 14971 is the standard for risk management of medical device software. The risk management report template should be modified to suit your company qms and. The following policy establishes criteria for risk acceptability following iso. The risk management report contains the output and summary of risk.
00021 REV01 ISO 149712019 Risk Management Process Overview
The following policy establishes criteria for risk acceptability following iso. The risk management report contains the output and summary of risk. The risk management report template should be modified to suit your company qms and. Here are all our posts. Iso 14971 is the standard for risk management of medical device software.
Case study — Risk management for medical devices (based on ISO 14971
The following policy establishes criteria for risk acceptability following iso. The risk management report contains the output and summary of risk. Here are all our posts. The risk management report template should be modified to suit your company qms and. Iso 14971 is the standard for risk management of medical device software.
ISO 14971 Risk Management Plan Template
Iso 14971 is the standard for risk management of medical device software. Here are all our posts. The risk management report contains the output and summary of risk. The following policy establishes criteria for risk acceptability following iso. The risk management report template should be modified to suit your company qms and.
What is new in ISO 149712019 Medical Device HQ
Iso 14971 is the standard for risk management of medical device software. Here are all our posts. The risk management report contains the output and summary of risk. The risk management report template should be modified to suit your company qms and. The following policy establishes criteria for risk acceptability following iso.
ISO 14971 Risk Management for Medical Devices [Guide]
Here are all our posts. The risk management report contains the output and summary of risk. Iso 14971 is the standard for risk management of medical device software. The following policy establishes criteria for risk acceptability following iso. The risk management report template should be modified to suit your company qms and.
ISO 149712019 Medical devices Application of Risk Management to
The following policy establishes criteria for risk acceptability following iso. Here are all our posts. The risk management report contains the output and summary of risk. Iso 14971 is the standard for risk management of medical device software. The risk management report template should be modified to suit your company qms and.
ISO 149712019 Changes in the Current Version of ISO 14971 Oriel
The following policy establishes criteria for risk acceptability following iso. The risk management report template should be modified to suit your company qms and. Here are all our posts. Iso 14971 is the standard for risk management of medical device software. The risk management report contains the output and summary of risk.
ISO 14971 Risk Management Plan Template [Free Download]
The risk management report template should be modified to suit your company qms and. The risk management report contains the output and summary of risk. The following policy establishes criteria for risk acceptability following iso. Iso 14971 is the standard for risk management of medical device software. Here are all our posts.
Here Are All Our Posts.
Iso 14971 is the standard for risk management of medical device software. The following policy establishes criteria for risk acceptability following iso. The risk management report template should be modified to suit your company qms and. The risk management report contains the output and summary of risk.
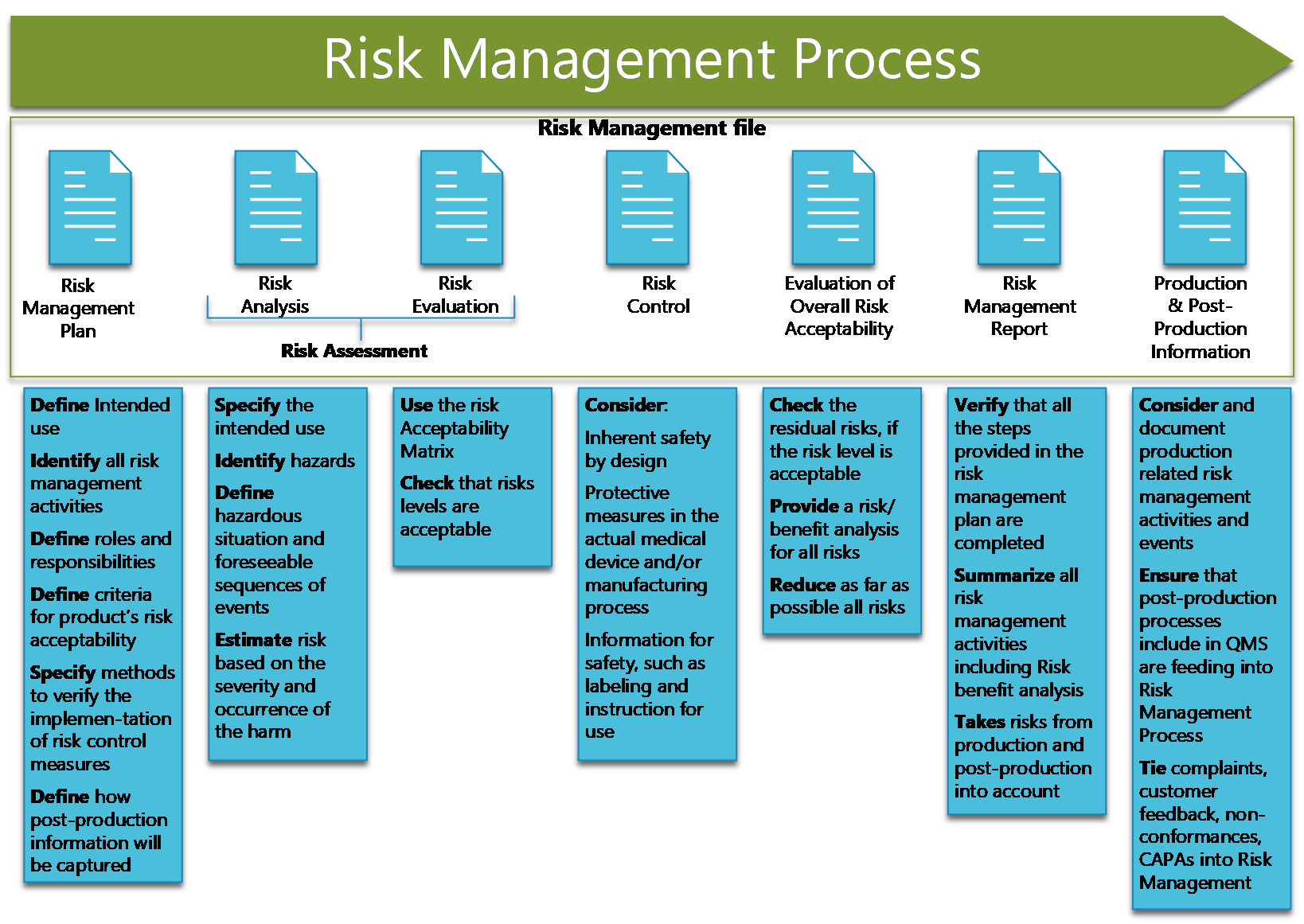
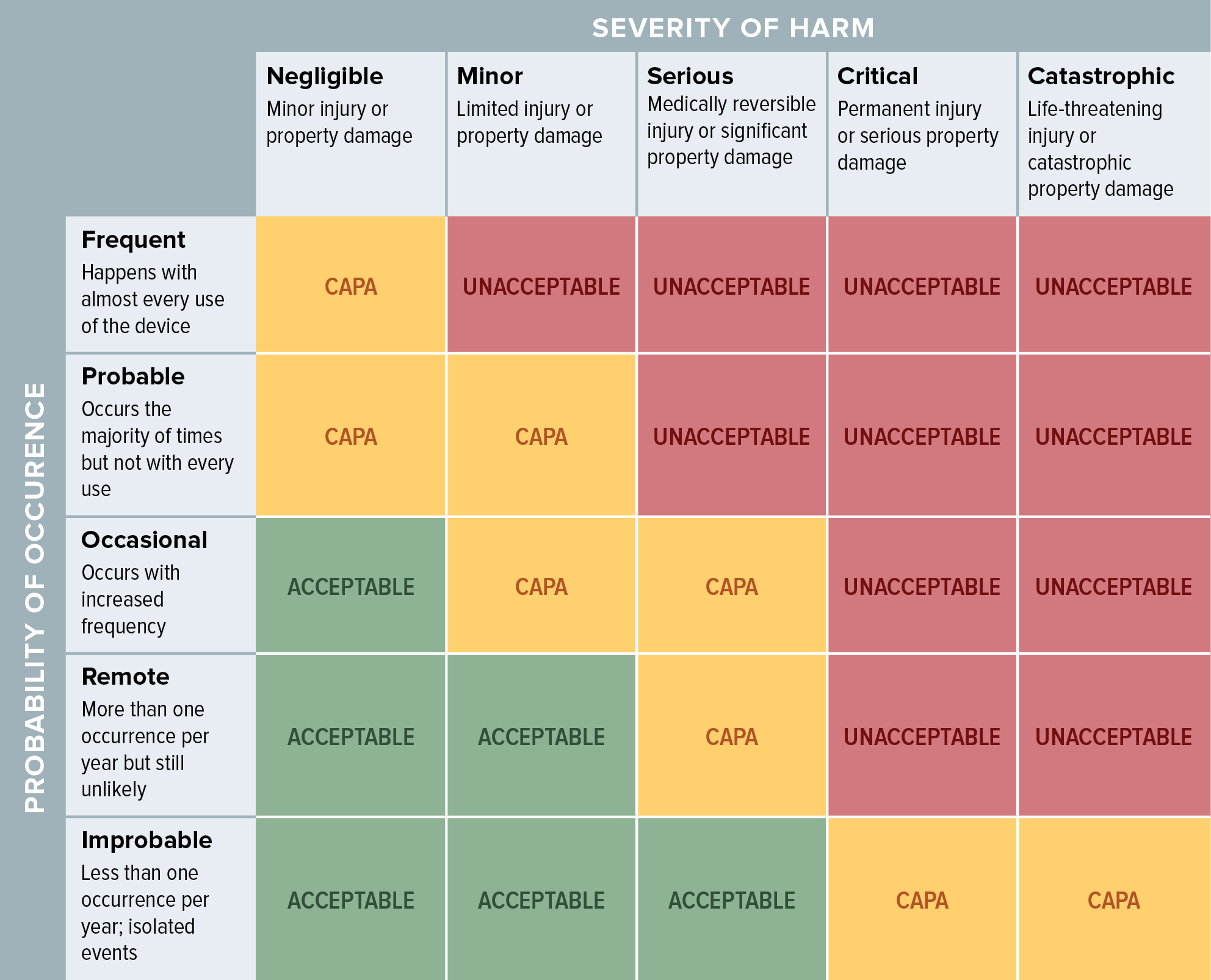
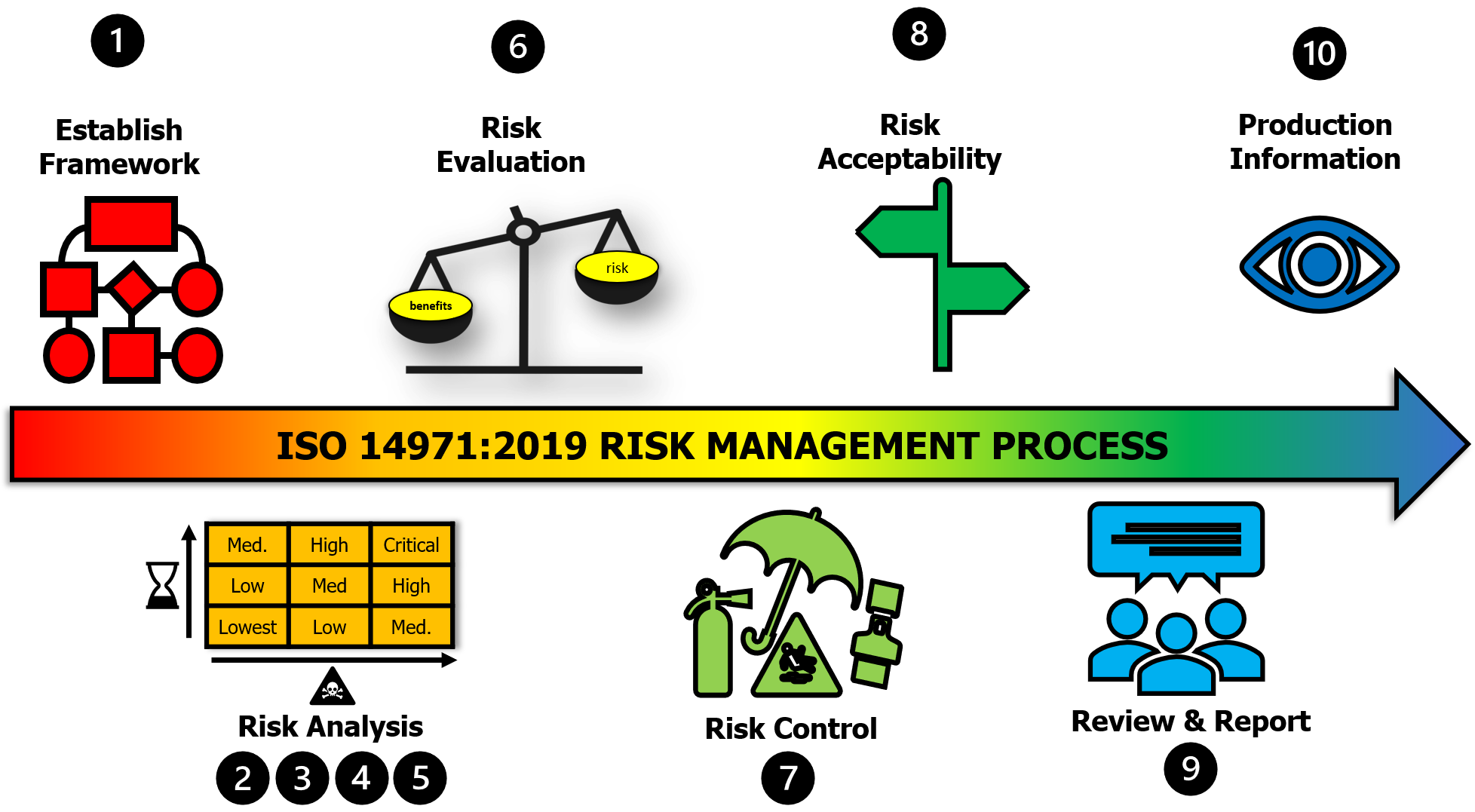



![ISO 14971 Risk Management for Medical Devices [Guide]](http://blog.greenlight.guru/hubfs/Blog-Giveaways/iso-14971-risk-management-process.png)
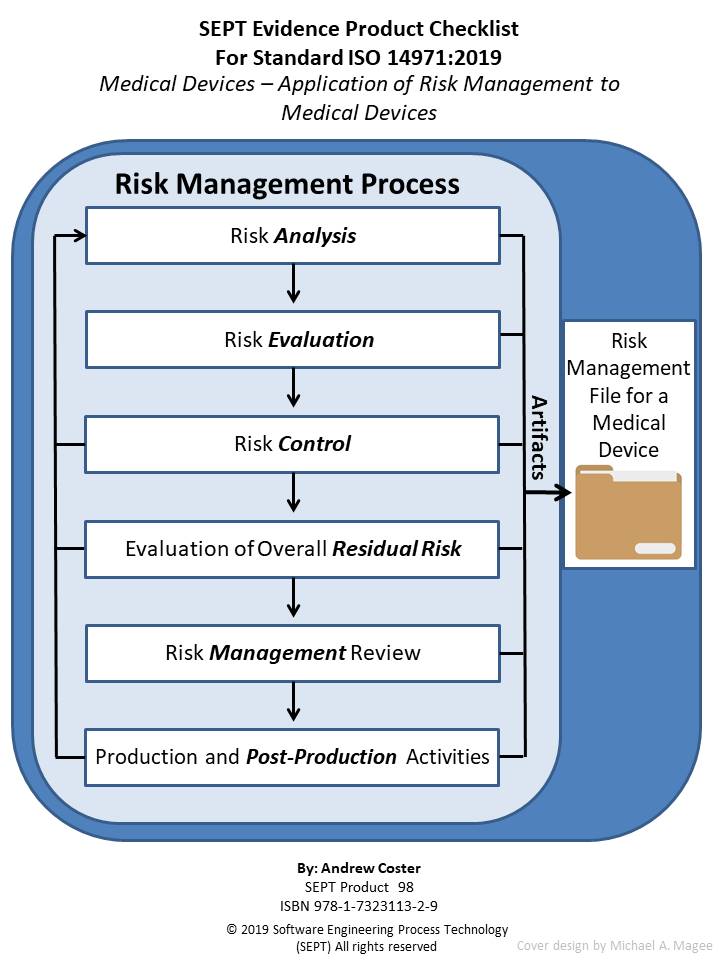
![ISO 14971 Risk Management Plan Template [Free Download]](https://www.greenlight.guru/hs-fs/hubfs/risk-management-template.png?width=600&name=risk-management-template.png)