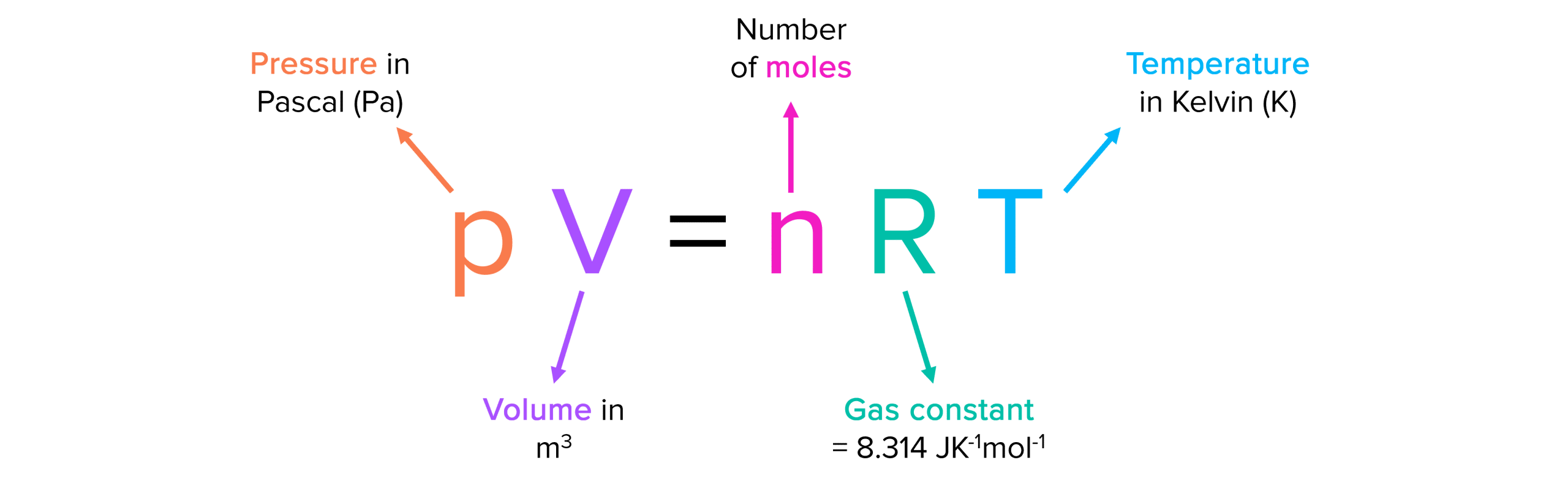Ideal Gas Law Worksheet Pdf - The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. The worksheet covers the gas laws,. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? A worksheet with questions and exercises on the ideal gas law, partial pressures and osmotic pressure. 10 ideal gas law 1.
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. The worksheet covers the gas laws,. 10 ideal gas law 1. A worksheet with questions and exercises on the ideal gas law, partial pressures and osmotic pressure.
The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. A worksheet with questions and exercises on the ideal gas law, partial pressures and osmotic pressure. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The worksheet covers the gas laws,. 10 ideal gas law 1.
7010641 Ideal Gas Law cdmellon LiveWorksheets
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The worksheet covers the gas laws,. 10 ideal gas law 1. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of.
Ideal gas law Chemistry of Physics Worksheet with Unit Conversions
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? A worksheet with questions and exercises on the ideal gas law, partial pressures and osmotic pressure. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is.
Ideal Gas Law Worksheet Carson Dellosa
The worksheet covers the gas laws,. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. 10 ideal gas law 1. How many moles of gas (air) are in the lungs of an adult with a.
Exercises Sections 10.3, 10.4 The Gas Laws; The IdealGas Equation
The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. The worksheet covers the gas laws,. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l?.
Ideal Gas Law Worksheet Answer Key —
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 10 ideal gas law 1. The worksheet covers the gas laws,. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of.
Ideal Gas Law Worksheet PVnRT Doc Template pdfFiller
10 ideal gas law 1. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? A.
Ideal Gas Law Worksheet Pv=nrt Answers
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? A worksheet with questions and exercises on the ideal gas law, partial pressures and osmotic pressure. The worksheet covers the gas laws,. 10 ideal gas law 1. The ideal gas law states that pv=nrt, where p is the pressure of.
SOLUTION Ideal gass law worksheet Studypool
The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 10 ideal gas law 1. The.
Ideal Gas Law Worksheet Answer Key Included Distance Learning
The worksheet covers the gas laws,. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 10 ideal gas law 1. A worksheet with questions and exercises on the ideal gas law, partial pressures and osmotic pressure. The ideal gas law states that pv=nrt, where p is the pressure of.
The Ideal Gas Equation MME
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The worksheet covers the gas laws,. 10 ideal gas law 1. A worksheet with questions and exercises on the ideal gas law, partial pressures and osmotic pressure. The ideal gas law states that pv=nrt, where p is the pressure of.
The Ideal Gas Law States That Pv=Nrt, Where P Is The Pressure Of A Gas, V Is The Volume Of The Gas, N Is The Number Of Moles Of Gas Present, R Is The.
How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 10 ideal gas law 1. The worksheet covers the gas laws,. A worksheet with questions and exercises on the ideal gas law, partial pressures and osmotic pressure.