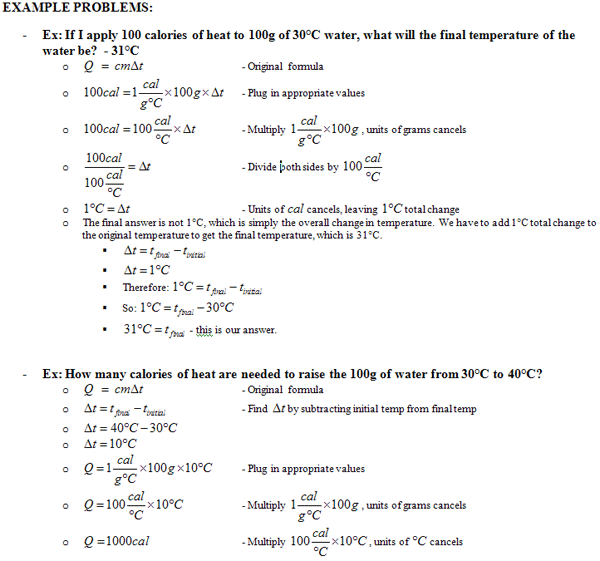
Heat Transfer Specific Heat Problems Worksheet - Heat transfer/ specific heat problems worksheet solving for heat (q) 1. Show all work and units. Caloric and joule’s discovery questions: How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oc to 18.0 oc? Specific heat, latent heat, phase change graphs, and calorimetry objective a: Use q = (m)(δt)(cp) to solve the following problems. How many joules of heat are required to raise the temperature of 550 g of. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Show all work and units. Heat transfer/ specific heat problems worksheet solving for heat (q) 1. How many joules of heat are required to raise the temperature of 550 g of. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems. Specific heat, latent heat, phase change graphs, and calorimetry objective a: How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oc to 18.0 oc? Caloric and joule’s discovery questions:
How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oc to 18.0 oc? Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Caloric and joule’s discovery questions: Heat transfer/ specific heat problems worksheet solving for heat (q) 1. Specific heat, latent heat, phase change graphs, and calorimetry objective a: Use q = (m)(δt)(cp) to solve the following problems. How many joules of heat are required to raise the temperature of 550 g of. Show all work and units.
Specific Heat Worksheets WorksheetsGO
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Use q = (m)(δt)(cp) to solve the following problems. Heat transfer/ specific heat problems worksheet solving for heat (q) 1. How many calories of heat are required to raise the temperature of 550 g of.
Heat Transfer Specific Heat Problems Worksheet —
Specific heat, latent heat, phase change graphs, and calorimetry objective a: Heat transfer/ specific heat problems worksheet solving for heat (q) 1. Show all work and units. Caloric and joule’s discovery questions: Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes.
Heat Transfer Specific Heat Problems Worksheet —
Caloric and joule’s discovery questions: How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oc to 18.0 oc? Show all work and units. Specific heat, latent heat, phase change graphs, and calorimetry objective a: Use q = (m)(δt)(cp) to solve the following problems.
Solved Heat Transfer/ Specific Heat Problems Worksheet
Show all work and units. Use q = (m)(δt)(cp) to solve the following problems. Heat transfer/ specific heat problems worksheet solving for heat (q) 1. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. How many joules of heat are required to raise the.
Specific Heat Worksheet Answers 1 Worksheets Library
Caloric and joule’s discovery questions: How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oc to 18.0 oc? Heat transfer/ specific heat problems worksheet solving for heat (q) 1. Specific heat, latent heat, phase change graphs, and calorimetry objective a: Calculate the specific heat capacity of a piece of wood if.
Heat Transfer Specific Heat Problems Worksheet
How many joules of heat are required to raise the temperature of 550 g of. Use q = (m)(δt)(cp) to solve the following problems. Specific heat, latent heat, phase change graphs, and calorimetry objective a: Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes..
Calculating Heat And Specific Heat Worksheets Answers
Use q = (m)(δt)(cp) to solve the following problems. Specific heat, latent heat, phase change graphs, and calorimetry objective a: How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oc to 18.0 oc? Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500.
Specific Heat Capacity Mixture Problems Worksheet
How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oc to 18.0 oc? Heat transfer/ specific heat problems worksheet solving for heat (q) 1. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g.
Specific Heat Capacity Practice Problems
Use q = (m)(δt)(cp) to solve the following problems. How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oc to 18.0 oc? Caloric and joule’s discovery questions: Heat transfer/ specific heat problems worksheet solving for heat (q) 1. How many joules of heat are required to raise the temperature of 550.
Specific Heat And Heat Capacity Worksheet
Caloric and joule’s discovery questions: How many joules of heat are required to raise the temperature of 550 g of. Show all work and units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. Heat transfer/ specific heat problems worksheet solving for heat (q).
Use Q = (M)(Δt)(Cp) To Solve The Following Problems.
Caloric and joule’s discovery questions: How many joules of heat are required to raise the temperature of 550 g of. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes. How many calories of heat are required to raise the temperature of 550 g of water from 12.0 oc to 18.0 oc?
Heat Transfer/ Specific Heat Problems Worksheet Solving For Heat (Q) 1.
Show all work and units. Specific heat, latent heat, phase change graphs, and calorimetry objective a: