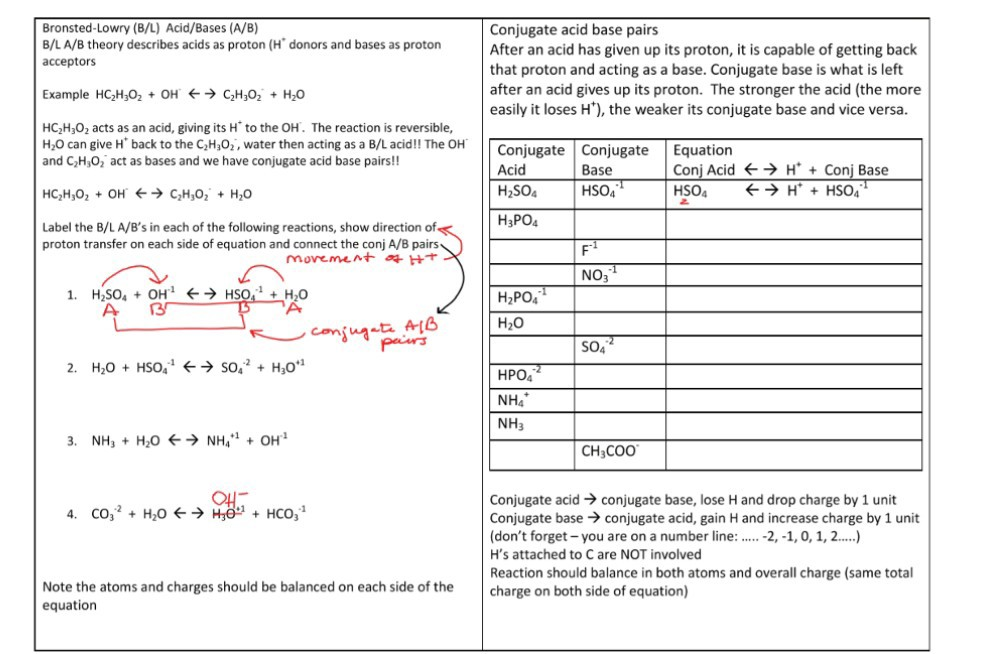
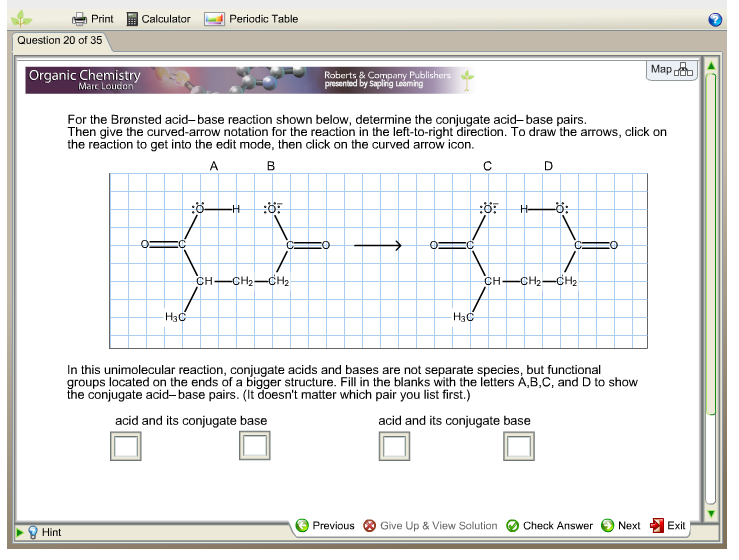
Conjugate Acid Base Pairs Chem Worksheet 19 2 - Write reactants and transfer a proton from the acid to the base: Label the acid, base, and conjugate acid and conjugate base. Since the product h 3o+. In the reaction above hf is the acid and h 2o is the base. Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a.
Write reactants and transfer a proton from the acid to the base: In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a. Since the product h 3o+. Label the acid, base, and conjugate acid and conjugate base. Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. In the reaction above hf is the acid and h 2o is the base.
In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a. Since the product h 3o+. In the reaction above hf is the acid and h 2o is the base. Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. Write reactants and transfer a proton from the acid to the base: Label the acid, base, and conjugate acid and conjugate base.
Fillable Online Conjugate Acid Base Pairs Chem Worksheet 192 Fax
In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a. Write reactants and transfer a proton from the acid to the base: Label the acid, base, and conjugate acid and conjugate base. Identify the acid, the base, the conjugate acid, and the conjugate.
10++ Conjugate Acid Base Pairs Worksheet Worksheets Decoomo
In the reaction above hf is the acid and h 2o is the base. Label the acid, base, and conjugate acid and conjugate base. Since the product h 3o+. Write reactants and transfer a proton from the acid to the base: Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations.
Conjugate Acid Base Pairs Name Chem Worksheet 192 PDF Acid
Label the acid, base, and conjugate acid and conjugate base. Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. In the reaction above hf is the acid and h 2o is the base. Since the product h 3o+. Write reactants and transfer a proton from the acid to the base:
Conjugate Acid Base Pairs Chem Worksheet 192
Label the acid, base, and conjugate acid and conjugate base. Since the product h 3o+. In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a. Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. In.
Conjugate Acid Base Pairs Chem Worksheet 192
Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. Write reactants and transfer a proton from the acid to the base: Label the acid, base, and conjugate acid and conjugate base. Since the product h 3o+. In the reaction above hf is the acid and h 2o is the base.
Conjugate Acid Base Pairs Chem Worksheet 192
Since the product h 3o+. In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a. Write reactants and transfer a proton from the acid to the base: In the reaction above hf is the acid and h 2o is the base. Identify the.
Conjugate Acid Base Pairs Chem Worksheet 192 Free Printable
Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a. In the reaction above hf is the acid and h 2o is the base. Since the.
Conjugate Acid Base Pairs Chem Worksheet 192 Answers Kayra Excel
Since the product h 3o+. Write reactants and transfer a proton from the acid to the base: Label the acid, base, and conjugate acid and conjugate base. Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. In the br nsted lowry definition of acids and bases a conjugate acid base pair consists.
Conjugate Acid Base Pairs Chem Worksheet 192
Since the product h 3o+. Write reactants and transfer a proton from the acid to the base: In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a. Label the acid, base, and conjugate acid and conjugate base. Identify the acid, the base, the.
Conjugate Acid Base Pairs Chem Worksheet 192
Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations. In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a. Write reactants and transfer a proton from the acid to the base: Label the acid, base,.
In The Reaction Above Hf Is The Acid And H 2O Is The Base.
In the br nsted lowry definition of acids and bases a conjugate acid base pair consists of two substances that differ only by the presence of a. Label the acid, base, and conjugate acid and conjugate base. Since the product h 3o+. Identify the acid, the base, the conjugate acid, and the conjugate base in each of the equations.